2019 Fry Trimeresurus Genus.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On Further Specimens of the Pit Viper Trimeresurus Erythrurus
Journal of Animal Diversity Online ISSN 2676-685X Volume 3, Issue 1 (2021) Research Article http://dx.doi.org/10.52547/JAD.2021.3.1.7 On further specimens of the Pit viper Trimeresurus erythrurus (Cantor, 1839) (Squamata: Viperidae), with description of a topotype and range extension to the Godavari Basin, peninsular India Kaushik Deutiˡ, Ramaswamy Aengals², Sujoy Rahaˡ, Sudipta Debnathˡ, Ponnusamy Sathiyaselvam3 and Sumaithangi Rajagopalan Ganesh4* 1Zoological Survey of India, Herpetology Division, 27 JL Nehru Road, Kolkata 700016, West Bengal, India ²Zoological Survey of India, Sunderbans Field Research Center, Canning 743329, West Bengal, India 3Bombay Natural History Society, Hornbill House, Shaheed Bhagat Singh Marg, Mumbai 400023, India 4Chennai Snake Park, Rajbhavan post, Chennai 600022, Tamil Nadu, India *Corresponding author : [email protected] Abstract We report on a topotypical specimen of the spot-tailed pit viper Trimeresurus erythrurus recorded from Sunderbans in India and a distant, southerly, range extension from Kakinada mangroves, based on preserved (n= 1, seen in 2019) and live uncollected (n= 2; seen in 2014) specimens, respectively. The specimens Received: 26 December 2020 (n= 3) share the following characteristics: verdant green dorsum, yellow iris, Accepted: 27 January 2021 white ventrolateral stripes in males, 23 midbody scale rows, 161–172 ventrals, Published online: 17 July 2021 61–76 subcaudals, and reddish tail tip. Drawing on the published records, its apparent rarity within its type locality and lack of records from the Circar Coast of India, our study significantly adds to the knowledge of the distribution and morphology of this species. Being a medically important venomous snake, its presence in the Godavari mangrove basin calls for wider dissemination of this information among medical practitioners, in addition to fundamental researchers like academics and herpetologists. -

Working with a Full Deck: the Use of Picture Cards in Herpetological Surveys of Timor-Leste
68 TECHNIQUES LITERATURE CITED NOUVELLET, P., G. S. A. RASMUSSEN, D. W. MACDONALD, AND F. COURCHAMP. 2012. Noisy clocks and silent sunrises: measurement methods of BURGER, B. 1988. Which way did he go? Telonics Quarterly 1:1. daily activity patterns. J. Zool., London 286:179–184. DORCAS, M. E., AND C. R. PETERSON. 2012. Automated data acquisi- RODDA, G. H. 1984. Movements of juvenile American crocodiles in Ga- tion. In R. W. McDiarmid, M. S. Foster, C. Guyer, J. Gibbons, and tun Lake, Panama. Herpetologica 40:444–451. N. Chernoff (eds.), Reptile Biodiversity: Standard Methods for In- ventory and Monitoring, pp. 61–68. University of California Press, Berkeley, California. Herpetological Review, 2013, 44(1), 68–76. © 2013 by Society for the Study of Amphibians and Reptiles Working with a Full Deck: the Use of Picture Cards in Herpetological Surveys of Timor-Leste Timor is the 44th largest island in the world and the seventh significantly, the Muséum National d’Histoire Naturelle in Paris, largest between Asia and Australia (area 29,402 km2). It occupies France; Naturalis, formerly the Rijksmuseum van Natuurlijke an extremely interesting geographical position within the bio- Historie, in Leiden, The Netherlands; and the Zoologisch Mu- geographical sub-region known as Wallacea, at the southeastern seum Amsterdam, now also housed in Leiden). Additional short edge of the Lesser Sunda Archipelago and separated from Aus- surveys were conducted there during the early 20th century (e.g., tralia by the Timor Sea (ca. 450 km). This gap was considerably Smith 1927; collections in the Natural History Museum, London, lessened during the final 250,000 years of the Pleistocene Epoch United Kingdom) and in the 1990s (e.g., How et al. -

Epidemiology of Snakebites from a General Hospital in Singapore: a 5-Year Retrospective Review (2004-2008) 1 Hock Heng Tan, MBBS, FRCS A&E (Edin), FAMS
640 Epidemiology of Snakebites—Hock Heng Tan Original Article Epidemiology of Snakebites from A General Hospital in Singapore: A 5-year Retrospective Review (2004-2008) 1 Hock Heng Tan, MBBS, FRCS A&E (Edin), FAMS Abstract Introduction: This is a retrospective study on the epidemiology of snakebites that were presented to an emergency department (ED) between 2004 and 2008. Materials and Methods: Snakebite cases were identified from International Classification of Diseases (ICD) code E905 and E906, as well as cases referred for eye injury from snake spit and records of antivenom use. Results: Fifty-two cases were identified: 13 patients witnessed the snake biting or spitting at them, 22 patients had fang marks and/or clinical features of envenomations and a snake was seen and the remaining 17 patients did not see any snake but had fang marks suggestive of snakebite. Most of the patients were young (mean age 33) and male (83%). The three most commonly identified snakes were cobras (7), pythons (4) and vipers (3). One third of cases occurred during work. Half of the bites were on the upper limbs and about half were on the lower limbs. One patient was spat in the eye by a cobra. Most of the patients (83%) arrived at the ED within 4 hours of the bite. Pain and swelling were the most common presentations. There were no significant systemic effects reported. Two patients had infection and 5 patients had elevated creatine kinase (>600U/L). Two thirds of the patients were admitted. One patient received antivenom therapy and 5 patients had some form of surgical intervention, of which 2 had residual disability. -

WHO Guidance on Management of Snakebites
GUIDELINES FOR THE MANAGEMENT OF SNAKEBITES 2nd Edition GUIDELINES FOR THE MANAGEMENT OF SNAKEBITES 2nd Edition 1. 2. 3. 4. ISBN 978-92-9022- © World Health Organization 2016 2nd Edition All rights reserved. Requests for publications, or for permission to reproduce or translate WHO publications, whether for sale or for noncommercial distribution, can be obtained from Publishing and Sales, World Health Organization, Regional Office for South-East Asia, Indraprastha Estate, Mahatma Gandhi Marg, New Delhi-110 002, India (fax: +91-11-23370197; e-mail: publications@ searo.who.int). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. In no event shall the World Health Organization be liable for damages arising from its use. -

Unusual Venom Phospholipases A2 of Two Primitive Tree Vipers Trimeresurus Puniceus and Trimeresurus Borneensis Ying-Ming Wang, Hao-Fan Peng and Inn-Ho Tsai
Unusual venom phospholipases A2 of two primitive tree vipers Trimeresurus puniceus and Trimeresurus borneensis Ying-Ming Wang, Hao-Fan Peng and Inn-Ho Tsai Institute of Biological Chemistry, Academia Sinica and Institute of Biochemical Sciences, National Taiwan University, Taipei, Taiwan Keywords To explore the venom diversity of Asian pit vipers, we investigated the phospholipase A ; phylogenetic analysis; 2 structure and function of venom phospholipase A2 (PLA2) derived from snake venom; Trimeresurus borneensis; two primitive tree vipers Trimeresurus puniceus and Trimeresurus borneen- Trimeresurus puniceus sis. We purified six novel PLA2s from T. puniceus venom and another three Correspondence from T. borneensis venom. All cDNAs encoding these PLA2s except one I.-H. Tsai, Institute of Biological Chemistry, were cloned, and the molecular masses and N-terminal sequences of the Academia Sinica and Institute of purified enzymes closely matched those predicted from the cDNA. Three Biochemical Sciences, National Taiwan contain K49 and lack a disulfide bond at C61–C91, in contrast with the University, PO Box 23-106, Taipei, D49-containing PLA2s in both venom species. They are less thermally Taiwan 10798 stable than other K49-PLA2s which contain seven disulfide bonds, as indi- Fax: +886 223635038 cated by a decrease of 8.8 °C in the melting temperature measured by CD E-mail: [email protected] spectroscopy. The M110D mutation in one of the K49-PLA2s apparently Note reduced its edematous potency. A phylogenetic tree based on the amino- Novel cDNA sequences encoding PLA2s acid sequences of 17 K49-PLA2s from Asian pit viper venoms illustrates have been submitted to EMBL Databank close relationships among the Trimeresurus species and intergeneric segre- and are available under accession numbers: gations. -

On Trimeresurus Sumatranus
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/266262458 On Trimeresurus sumatranus (Raffles, 1822), with the designation of a neotype and the description of a new species of pitviper from Sumatra (Squamata: Viperidae: Crotalinae) Article in Amphibian and Reptile Conservation · September 2014 CITATIONS READS 4 360 3 authors, including: Gernot Vogel Irvan Sidik Independent Researcher Indonesian Institute of Sciences 102 PUBLICATIONS 1,139 CITATIONS 12 PUBLICATIONS 15 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Save Vietnam Biodiversity View project Systematics of the genus Pareas View project All content following this page was uploaded by Gernot Vogel on 01 October 2014. The user has requested enhancement of the downloaded file. Comparative dorsal view of the head of Trimeresurus gunaleni spec. nov. (left) and T. sumatranus (right). Left from above: male, female (holotype), male, all alive, from Sumatra Utara Province, Sumatra. Right: adult female alive from Bengkulu Province, Su- matra, adult male alive from Bengkulu Province, Sumatra, preserved female from Borneo. Photos: N. Maury. Amphib. Reptile Conserv. | amphibian-reptile-conservation.org (1) September 2014 | Volume 8 | Number 2 | e80 Copyright: © 2014 Vogel et al. This is an open-access article distributed under the terms of the Creative Commons Attribution–NonCommercial–NoDerivs 3.0 Unported License, Amphibian & Reptile Conservation which permits -

Venom Protein of the Haematotoxic Snakes Cryptelytrops Albolabris
S HORT REPORT ScienceAsia 37 (2011): 377–381 doi: 10.2306/scienceasia1513-1874.2011.37.377 Venom protein of the haematotoxic snakes Cryptelytrops albolabris, Calloselasma rhodostoma, and Daboia russelii siamensis Orawan Khow, Pannipa Chulasugandha∗, Narumol Pakmanee Research and Development Department, Queen Saovabha Memorial Institute, Patumwan, Bangkok 10330 Thailand ∗Corresponding author, e-mail: pannipa [email protected] Received 1 Dec 2010 Accepted 6 Sep 2011 ABSTRACT: The protein concentration and protein pattern of crude venoms of three major haematotoxic snakes of Thailand, Cryptelytrops albolabris (green pit viper), Calloselasma rhodostoma (Malayan pit viper), and Daboia russelii siamensis (Russell’s viper), were studied. The protein concentrations of all lots of venoms studied were comparable. The chromatograms, from reversed phase high performance liquid chromatography, of C. albolabris venom and C. rhodostoma venom were similar but they were different from the chromatogram of D. r. siamensis venom. C. rhodostoma venom showed the highest number of protein spots on 2-dimensional gel electrophoresis (pH gradient 3–10), followed by C. albolabris venom and D. r. siamensis venom, respectively. The protein spots of C. rhodostoma venom were used as reference proteins in matching for similar proteins of haematotoxic snakes. C. albolabris venom showed more similar protein spots to C. rhodostoma venom than D. r. siamensis venom. The minimum coagulant dose could not be determined in D. r. siamensis venom. KEYWORDS: 2-dimensional gel electrophoresis, reverse phase high performance liquid chromatography, minimum coag- ulant dose INTRODUCTION inducing defibrination 5–7. The venom of D. r. sia- mensis directly affects factor X and factor V of the In Thailand there are 163 snake species, 48 of which haemostatic system 8,9 . -

NHBSS 061 1G Hikida Fieldg
Book Review N$7+IST. BULL. S,$0 SOC. 61(1): 41–51, 2015 A Field Guide to the Reptiles of Thailand by Tanya Chan-ard, John W. K. Parr and Jarujin Nabhitabhata. Oxford University Press, New York, 2015. 344 pp. paper. ISBN: 9780199736492. 7KDLUHSWLOHVZHUHÀUVWH[WHQVLYHO\VWXGLHGE\WZRJUHDWKHUSHWRORJLVWV0DOFROP$UWKXU 6PLWKDQG(GZDUG+DUULVRQ7D\ORU7KHLUFRQWULEXWLRQVZHUHSXEOLVKHGDV6MITH (1931, 1935, 1943) and TAYLOR 5HFHQWO\RWKHUERRNVDERXWUHSWLOHVDQGDPSKLELDQV LQ7KDLODQGZHUHSXEOLVKHG HJ&HAN-ARD ET AL., 1999: COX ET AL DVZHOODVPDQ\ SDSHUV+RZHYHUWKHVHERRNVZHUHWD[RQRPLFVWXGLHVDQGQRWJXLGHVIRURUGLQDU\SHRSOH7ZR DGGLWLRQDOÀHOGJXLGHERRNVRQUHSWLOHVRUDPSKLELDQVDQGUHSWLOHVKDYHDOVREHHQSXEOLVKHG 0ANTHEY & GROSSMANN, 1997; DAS EXWWKHVHERRNVFRYHURQO\DSDUWRIWKHIDXQD The book under review is very well prepared and will help us know Thai reptiles better. 2QHRIWKHDXWKRUV-DUXMLQ1DEKLWDEKDWDZDVP\ROGIULHQGIRUPHUO\WKH'LUHFWRURI1DWXUDO +LVWRU\0XVHXPWKH1DWLRQDO6FLHQFH0XVHXP7KDLODQG+HZDVDQH[FHOOHQWQDWXUDOLVW DQGKDGH[WHQVLYHNQRZOHGJHDERXW7KDLDQLPDOVHVSHFLDOO\DPSKLELDQVDQGUHSWLOHV,Q ZHYLVLWHG.KDR6RL'DR:LOGOLIH6DQFWXDU\WRVXUYH\KHUSHWRIDXQD+HDGYLVHGXV WRGLJTXLFNO\DURXQGWKHUH:HFROOHFWHGIRXUVSHFLPHQVRIDibamusZKLFKZHGHVFULEHG DVDQHZVSHFLHVDibamus somsaki +ONDA ET AL 1RZ,DPYHU\JODGWRNQRZWKDW WKLVERRNZDVSXEOLVKHGE\KLPDQGKLVFROOHDJXHV8QIRUWXQDWHO\KHSDVVHGDZD\LQ +LVXQWLPHO\GHDWKPD\KDYHGHOD\HGWKHSXEOLFDWLRQRIWKLVERRN7KHERRNLQFOXGHVQHDUO\ DOOQDWLYHUHSWLOHV PRUHWKDQVSHFLHV LQ7KDLODQGDQGPRVWSLFWXUHVZHUHGUDZQZLWK H[FHOOHQWGHWDLO,WLVDYHU\JRRGÀHOGJXLGHIRULGHQWLÀFDWLRQRI7KDLUHSWLOHVIRUVWXGHQWV -

The Herpetological Journal
Volume 14, Number 1 January 2004 ISSN 0268-0130 THE HERPETOLOGICAL JOURNAL Published by the Indexed in BRITISH HERPETOLOGJCAL SOCIETY Current Contents HERPETOLOGICAL JOURNAL, Vol. 14, pp. 21-33 (2004) REASSESSMENT OF THE VALIDITY AND DIAGNOSIS OF THE PITVIPER TRIMERESUR US VE NUSTUS VOGEL, 1991 ANITA MALHOTRA AND ROGER S. THORPE School of Biological Sciences, University of Wa les Bangor, Bangor, UK Trimeresurus venustus Vogel, I 99 I was described from southern Thailand in I 991 ' and distinguished from the similar T. kanburiensis primarily by the following characters: 21 scale rows at midbody rather than I 9 and less irregular and indented supraoculars. However, very few specimens of T. kanburiensis were known at the time of this description, and the name T. venustus has not been universally accepted. Recently, live specimens from the type locality of T. kanburiensis in western Th ailand have become available, allowing a reassessment of the status -of the southern Thai population. Phylogenetic analysis of two mitochondrial gene regions indicated that specimens from south Thailand are genetically quite distinct from the specimen from the type locality, and the former are more closely related to T. macrops than to T. kanburiensis. We present a multivariate morphometric analysis of the six specimens of T. kanburiensis from the type locality that are now known and twenty specimens from southern Thai land. Despite the small sample size, it is clear that some of the diagnostic characteristics used to define T. venustus are invalid. We conclude that the current evidence indicates that T. venustus is a valid species, and present new diagnostic characters to separate it from T. -

Venomics of Trimeresurus (Popeia) Nebularis, the Cameron Highlands Pit Viper from Malaysia: Insights Into Venom Proteome, Toxicity and Neutralization of Antivenom
toxins Article Venomics of Trimeresurus (Popeia) nebularis, the Cameron Highlands Pit Viper from Malaysia: Insights into Venom Proteome, Toxicity and Neutralization of Antivenom Choo Hock Tan 1,*, Kae Yi Tan 2 , Tzu Shan Ng 2, Evan S.H. Quah 3 , Ahmad Khaldun Ismail 4 , Sumana Khomvilai 5, Visith Sitprija 5 and Nget Hong Tan 2 1 Department of Pharmacology, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia; 2 Department of Molecular Medicine, Faculty of Medicine, University of Malaya, 50603 Kuala Lumpur, Malaysia; [email protected] (K.Y.T.); [email protected] (T.S.N.); [email protected] (N.H.T.) 3 School of Biological Sciences, Universiti Sains Malaysia, 11800 Minden, Penang, Malaysia; [email protected] 4 Department of Emergency Medicine, Universiti Kebangsaan Malaysia Medical Centre, 56000 Kuala Lumpur, Malaysia; [email protected] 5 Thai Red Cross Society, Queen Saovabha Memorial Institute, Bangkok 10330, Thailand; [email protected] (S.K.); [email protected] (V.S.) * Correspondence: [email protected] Received: 31 December 2018; Accepted: 30 January 2019; Published: 6 February 2019 Abstract: Trimeresurus nebularis is a montane pit viper that causes bites and envenomation to various communities in the central highland region of Malaysia, in particular Cameron’s Highlands. To unravel the venom composition of this species, the venom proteins were digested by trypsin and subjected to nano-liquid chromatography-tandem mass spectrometry (LC-MS/MS) for proteomic profiling. Snake venom metalloproteinases (SVMP) dominated the venom proteome by 48.42% of total venom proteins, with a characteristic distribution of P-III: P-II classes in a ratio of 2:1, while P-I class was undetected. -

Status and Diversity of Snakes (Reptilia: Squamata: Serpentes) at the Chittagong University Campus in Chittagong
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 November 2015 | 7(14): 8159–8166 Status and diversity of snakes (Reptilia: Squamata: Serpentes) at the Chittagong University Campus in Chittagong, Bangladesh ISSN 0974-7907 (Online) ISSN 0974-7893 (Print) Communication Short M.F. Ahsan 1, I.K.A. Haidar 2 & M.M. Rahman 3 OPEN ACCESS 1 Professor, 2,3 Student, Department of Zoology, University of Chittagong, Chittagong 4331, Bangladesh 1 [email protected] (corresponding author), 2 [email protected], 3 [email protected] Abstract: A study was conducted on the status and diversity of snakes Most of the snakes are harmless and even beneficial of the Chittagong University Campus (CUC) between September 2013 to humans and to the natural ecosystem. They are good and December 2014, and on preserved snake specimens of museums of CUC (Department of Zoology, University of Chittagong; Institute of friends of farmers and help in maintaining the ecological Marine Sciences and Fisheries, University of Chittagong; and Institute balance. Snakes are found all over the world except the of Forestry and Environmental Sciences, University of Chittagong). Thirty-six species of snakes belonging to 22 genera and five families Arctic Region, New Zealand and Ireland (Goin & Goin (Typhlopidae, Pythonidae, Colubridae, Elapidae and Viperidae) were 1971). There are about 3,496 species of snakes under recorded from CUC during the study period. Colubridae comprised the 26 families around the world (Uetz & Hošek 2015). highest (24 species i.e., 66.67%) number of species and Pythonidae the lowest (1 species). Checkered Keelback Xenochrophis piscator Snakes of Bangladesh are still poorly known. -
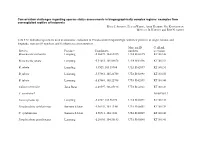
Conservation Challenges Regarding Species Status Assessments in Biogeographically Complex Regions: Examples from Overexploited Reptiles of Indonesia KYLE J
Conservation challenges regarding species status assessments in biogeographically complex regions: examples from overexploited reptiles of Indonesia KYLE J. SHANEY, ELIJAH WOSTL, AMIR HAMIDY, NIA KURNIAWAN MICHAEL B. HARVEY and ERIC N. SMITH TABLE S1 Individual specimens used in taxonomic evaluation of Pseudocalotes tympanistriga, with their province of origin, latitude and longitude, museum ID numbers, and GenBank accession numbers. Museum ID GenBank Species Province Coordinates numbers accession Bronchocela cristatella Lampung -5.36079, 104.63215 UTA R 62895 KT180148 Bronchocela jubata Lampung -5.54653, 105.04678 UTA R 62896 KT180152 B. jubata Lampung -5.5525, 105.18384 UTA R 62897 KT180151 B. jubata Lampung -5.57861, 105.22708 UTA R 62898 KT180150 B. jubata Lampung -5.57861, 105.22708 UTA R 62899 KT180146 Calotes versicolor Jawa Barat -6.49597, 106.85198 UTA R 62861 KT180149 C. versicolor* NC009683.1 Gonocephalus sp. Lampung -5.2787, 104.56198 UTA R 60571 KT180144 Pseudocalotes cybelidermus Sumatra Selatan -4.90149, 104.13401 UTA R 60551 KT180139 P. cybelidermus Sumatra Selatan -4.90711, 104.1348 UTA R 60549 KT180140 Pseudocalotes guttalineatus Lampung -5.28105, 104.56183 UTA R 60540 KT180141 P. guttalineatus Sumatra Selatan -4.90681, 104.13457 UTA R 60501 KT180142 Pseudocalotes rhammanotus Lampung -4.9394, 103.85292 MZB 10804 KT180147 Pseudocalotes species 4 Sumatra Barat -2.04294, 101.31129 MZB 13295 KT211019 Pseudocalotes tympanistriga Jawa Barat -6.74181, 107.0061 UTA R 60544 KT180143 P. tympanistriga Jawa Barat -6.74181, 107.0061 UTA R 60547 KT180145 Pogona vitticeps* AB166795.1 *Entry to GenBank by previous authors TABLE S2 Reptile species currently believed to occur Java and Sumatra, Indonesia, with IUCN Red List status, and certainty of occurrence.