Host Specificity of Microsporidia Pathogenic to the Gypsy Moth
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
732 _____________Mun. Ent. Zool. Vol. 7, No. 2, June 2012__________ STRUCTURE OF LEPIDOPTEROCENOSES ON OAKS QUERCUS DALECHAMPII AND Q. CERRIS IN CENTRAL EUROPE AND ESTIMATION OF THE MOST IMPORTANT SPECIES Miroslav Kulfan* * Department of Ecology, Faculty of Natural Sciences, Comenius University, Mlynská dolina B-1, SK-84215 Bratislava, SLOVAKIA. E-mail: [email protected] [Kulfan, M. 2012. Structure of lepidopterocenoses on oaks Quercus dalechampii and Q. cerris in Central Europe and estimation of the most important species. Munis Entomology & Zoology, 7 (2): 732-741] ABSTRACT: On the basis of lepidopterous larvae a total of 96 species on Quercus dalechampii and 58 species on Q. cerris were recorded in 10 study plots of Malé Karpaty and Trnavská pahorkatina hills. The families Geometridae, Noctuidae and Tortricidae encompassed the highest number of found species. The most recorded species belonged to the trophic group of generalists. On the basis of total abundance of lepidopterous larvae found on Q. dalechampii from all the study plots the most abundant species was evidently Operophtera brumata. The most abundant species on Q. cerris was Cyclophora ruficiliaria. Based on estimated oak leaf area consumed by a larva it is shown that Lymantria dispar was the most important leaf-chewing species of both Q. dalechampii and Q. cerris. KEY WORDS: Slovakia, Quercus dalechampii, Q. cerris, the most important species. About 300 Lepidoptera species are known to damage the assimilation tissue of oaks in Central Europe (Patočka, 1954, 1980; Patočka et al.1999; Reiprich, 2001). Lepidoptera larvae are shown to be the most important group of oak defoliators (Patočka et al., 1962, 1999). -

Thesis.Pdf (3.979Mb)
FACULTY OF BIOSCIENCES, FISHERIES AND ECONOMICS DEPARTMENT OF ARCTIC AND MARINE BIOLOGY Cyclically outbreaking geometrid moths in sub-arctic mountain birch forest: the organization and impacts of their interactions with animal communities — Ole Petter Laksforsmo Vindstad A dissertation for the degree of Philosophiae Doctor – October 2014 Cyclically outbreaking geometrid moths in sub-arctic mountain birch forest: the organization and impacts of their interactions with animal communities Ole Petter Laksforsmo Vindstad A dissertation for the degree of Philosophiae Doctor University of Tromsø – The arctic university of Norway Faculty of Biosciences, Fisheries and Economics Department of Arctic and Marine Biology Autumn 2014 1 Dedicated to everyone who has helped me along the way 2 Supervisors Professor Rolf Anker Ims1 Senior researcher Jane Uhd Jepsen2 1 Department of Arctic and Marine Biology, University of Tromsø, Tromsø, Norway 2 Norwegian Institute for Nature Research, Fram Centre, Tromsø, Norway Cover photos Front cover – Larvae of Epirrita autumnata feeding on mountain birch during a moth outbreak in northern Norway. Photo: Moritz Klinghardt Study I – Portrait of Agrypon flaveolatum. One of the most important larval parasitoid species in study I. Photo: Ole Petter Laksforsmo Vindstad Study II – Carcass of an Operophtera brumata larva, standing over the cocoon of its killer, the parasitoid group Protapanteles anchisiades/P. immunis/Cotesia salebrosa. Photo: Ole Petter Laksforsmo Vindstad Study III – Larva of the parasitoid group Phobocampe sp./Sinophorus crassifemur emerging from Agriopis aurantiaria host larva. Photo: Tino Schott Study IV – An area of healthy mountain birch forest, representative for the undamaged sampling sites in study IV and V. Photo: Jakob Iglhaut Study V – An area of mountain birch forest that has been heavily damaged by a moth outbreak, representative for the damaged sampling sites in study IV and V. -

Contributions to Knowledge of the Geometrid Fauna of Bulgaria and Greece, with Four Species New for the Greek Fauna (Lepidoptera: Geometridae) (Plate 12)
Esperiana Band 18: 221- 224 Bad Staffelstein; Schwanfeld, 02. Dezember 2013 ISBN 978-3-938249-04-8 Contributions to knowledge of the geometrid fauna of Bulgaria and Greece, with four species new for the Greek fauna (Lepidoptera: Geometridae) (plate 12) Balázs TÓTH, János BABICS & Balázs BENEDEK Abstract During a tour led by the authors to Bulgaria and Greece in March, 2013, a total of 19 geometrid species were observed at five localities. Biston achyra WEHRLI, 1936, Agriopis marginaria (FABRICIUS, 1776), A. leucophaearia ([DENIS & SCHIffERMÜLLER], 1775) and Erannis ankeraria (STAUDINGER, 1861) were found for the first time in Greece. An entirely new habitat type is included to the biotope range of E. ankeraria, arousing the possibility of this species being widespread in the Mediterranean countries. The authors hope that these observations will encourage attention to the exploration of the populations, thereby contributing to the more efficient protection of this species. Checklists are given to each collecting events. Key words: Biston, Agriopis, Erannis, Bulgaria, Greece, new data, oak woodland, macchia-scrub, soil types Introduction Biston achyra WEHRLI, 1936 was described from Asia Minor and subsequently found in Ukraine (KOSTJUK, 1990), the Levant (KOSTJUK, 1991) and Russia (SINEV, 2008). This species can be distinguished from its relative B. strataria (HUFNAGEL, 1767) by its considerably smaller size, more elongated forewing, and the presence of discal spot on the hindwing. Agriopis marginaria (FABRICIUS, 1776) and A. leucophaearia ([DENIS & SCHIFFERMÜLLER], 1775) are both frequent and widespread in Europe. The former species is distributed from the Iberian Peninsula to the Urals and the Caucasus Mts., and is also present in Asia Minor. -

JAVELIN WG® Spray Must Be Deposited at the Larval Feeding Site
For Control of Insect Pests of Vegetables, Fruit and Field Crops ACTIVE INGREDIENT: Bacillus thuringiensis, subspecies kurstaki strain SA-11 solids, spores, and Lepidopteran active toxins* .............................................................................. 85.0% OTHER INGREDIENTS: ........................................................................................ 15.0% TOTAL 100.0% * The percentage active ingredient does not indicate product performance and potency measurements are not federally standardized. KEEP OUT OF REACH OF CHILDREN CAUTION See additional precautionary statements EPA REG. NO.: 70051-66 Lot No. EPA EST. NO.: Net Contents: Manufactured by: Certis USA LLC ESL 20160825 9145 Guilford Road, Suite 175 rev20191112 Columbia, MD 21046 This is a Specimen Label. It may not reflect the most-recent approved label for use in your state. Always refer to the label on the product packaging for approved use instructions. Please contact your Certis sales representative for more information. Page 1 of 17 PRECAUTIONARY STATEMENTS HAZARDS TO HUMANS AND DOMESTIC ANIMALS CAUTION. Harmful if absorbed through skin. Avoid contact with skin, eyes or clothing. Wash thoroughly with soap and water after handling and before eating, drinking, chewing gum, using tobacco, or using the toilet. Remove and wash contaminated clothing before reuse. Harmful if inhaled. Avoid breathing spray mist. Prolonged or frequently repeated skin contact may cause allergic reactions in some individuals. FIRST AID If on skin or clothing: Take off contaminated clothing. Rinse skin immediately with plenty of water for 15-20 minutes. If in eyes: Hold eye open and rinse slowly and gently with water for 15-20 minutes. Remove contact lenses, if present, after the first 5 minutes, then continue rinsing eye. If inhaled: Move person to fresh air. -

Lepidoptera in Cheshire in 2002
Lepidoptera in Cheshire in 2002 A Report on the Micro-Moths, Butterflies and Macro-Moths of VC58 S.H. Hind, S. McWilliam, B.T. Shaw, S. Farrell and A. Wander Lancashire & Cheshire Entomological Society November 2003 1 1. Introduction Welcome to the 2002 report on lepidoptera in VC58 (Cheshire). This is the second report to appear in 2003 and follows on from the release of the 2001 version earlier this year. Hopefully we are now on course to return to an annual report, with the 2003 report planned for the middle of next year. Plans for the ‘Atlas of Lepidoptera in VC58’ continue apace. We had hoped to produce a further update to the Atlas but this report is already quite a large document. We will, therefore produce a supplementary report on the Pug Moths recorded in VC58 sometime in early 2004, hopefully in time to be sent out with the next newsletter. As usual, we have produced a combined report covering micro-moths, macro- moths and butterflies, rather than separate reports on all three groups. Doubtless observers will turn first to the group they are most interested in, but please take the time to read the other sections. Hopefully you will find something of interest. Many thanks to all recorders who have already submitted records for 2002. Without your efforts this report would not be possible. Please keep the records coming! This request also most definitely applies to recorders who have not sent in records for 2002 or even earlier. It is never too late to send in historic records as they will all be included within the above-mentioned Atlas when this is produced. -

A-Razowski X.Vp:Corelventura
Acta zoologica cracoviensia, 46(3): 269-275, Kraków, 30 Sep., 2003 Reassessment of forewing pattern elements in Tortricidae (Lepidoptera) Józef RAZOWSKI Received: 15 March, 2003 Accepted for publication: 20 May, 2003 RAZOWSKI J. 2003. Reassessment of forewing pattern elements in Tortricidae (Lepidop- tera). Acta zoologica cracoviensia, 46(3): 269-275. Abstract. Forewing pattern elements of moths in the family Tortricidae are discussed and characterized. An historical review of the terminology is provided. A new system of nam- ing pattern elements is proposed. Key words. Lepidoptera, Tortricidae, forewing pattern, analysis, terminology. Józef RAZOWSKI, Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, S³awkowska 17, 31-016 Kraków, Poland. E-mail: razowski.isez.pan.krakow.pl I. INTRODUCTION Early tortricid workers such as HAWORTH (1811), HERRICH-SCHHÄFFER (1856), and others pre- sented the first terminology for forewing pattern elements in their descriptions of new species. Nearly a century later, SÜFFERT (1929) provided a more eclectic discussion of pattern elements for Lepidoptera in general. In recent decades, the common and repeated use of specific terms in de- scriptions and illustrations by FALKOVITSH (1966), DANILEVSKY and KUZNETZOV (1968), and oth- ers reinforced these terms in Tortricidae. BRADLEY et al. (1973) summarized and commented on all the English terms used to describe forewing pattern elements. DANILEVSKY and KUZNETZOV (1968) and KUZNETZOV (1978) analyzed tortricid pattern elements, primarily Olethreutinae, dem- onstrating the taxonomic significance of the costal strigulae in that subfamily. For practical pur- poses they numbered the strigulae from the forewing apex to the base, where the strigulae often become indistinct. KUZNETZOV (1978) named the following forewing elements in Tortricinae: ba- sal fascia, subterminal fascia, outer fascia (comprised of subapical blotch and outer blotch), apical spot, and marginal line situated in the marginal fascia (a component of the ground colour). -

Und Herbst-Noctuidenfauna Von Nordgriechenland (Lepidoptera, Noctuidae)
Esperiana Band 16: 39-65 Schwanfeld, 06. Dezember 2011 ISBN 978-3-938249-01-7 Zweiter Beitrag zur Frühjahrs- und Herbst-Noctuidenfauna von Nordgriechenland (Lepidoptera, Noctuidae) Hartmut WEGNER Inhalt: In den Jahren 2002 – 2006 wurden fünf weitere Reisen zur Dokumentation der Noctuidenfauna in Nord-Griechenland durchgeführt: vier Reisen im Herbst, eine Reise im Frühjahr. Die Ergebnisse der Reisen werden dargestellt. Kommentiert werden besonders die Arten, zu deren Verbreitung, Abundanz und Bionomie erweiterte Erkenntnisse erzielt wurden. Bisher unbekannte oder nicht fotografisch abgebildete Raupen werden dargestellt und kurz beschrieben:Conistra ragusae macedo- nica, Lithophane merckii, Griposia pinkeri, Griposia wegneri. Neu für die Fauna Griechenlands wurden Agrochola luteogrisea, Conistra iana, Lithophane furcifera und Lithophane leautieri festgestellt. Abstract: In the years 2002 to 2006, further five trips to Northern Greece took place with the purpose to document the Noc- tuidae fauna: Four trips in autumn, one trip in spring time. The results of these trips are illustrated. Especially commented are those species to whose spread, abundance and bionomics enhanced findings were achieved. Caterpillars unknown or not photo-optically pictured so far are shown and briefly described:Conistra ragusae macedonica, Lithophane merckii, Griposia pinkeri, Griposia wegneri. Newly discovered in the fauna of Greece are Agrochola luteogrisea, Conistra iana, Lithophane furcifera and Lithophane leautieri. Einleitung In Fortsetzung der Reisen 1998 – 2001 (WEGNER 2002) wurden 2002 – 2006 fünf weitere Reisen zur Erfassung der Noctuidenfauna Nord-Griechenlands durchgeführt. Die Untersuchungen fanden schwerpunktmäßig in der Provinz Epirus im Bergland von Zagori, in der Provinz Dytiki Makedonia in der Umgebung von Kosani und in der Provinz Anatoliki Makedonia (Thrakien) im Hügelland der Umgebung von Alexandroupoli statt. -

Lepidoptera: Noctuidae, Psaphidinae) SHILAP Revista De Lepidopterología, Vol
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Guerrero, J. J.; Garre, M.; Rubio, R. M.; Ortiz, A. S. Allophyes corsica (Spuler, 1908) nueva especie para la fauna de España (Lepidoptera: Noctuidae, Psaphidinae) SHILAP Revista de Lepidopterología, vol. 42, núm. 168, diciembre, 2014, pp. 615-618 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Disponible en: http://www.redalyc.org/articulo.oa?id=45540983008 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto 615-618 Allophyes corsica (Spul 26/11/14 11:10 Página 615 SHILAP Revta. lepid., 42 (168), diciembre 2014: 615-618 eISSN: 2340-4078 ISSN: 0300-5267 Allophyes corsica (Spuler, 1908) nueva especie para la fauna de España (Lepidoptera: Noctuidae, Psaphidinae) J. J. Guerrero, M. Garre, R. M. Rubio & A. S. Ortiz Resumen Se cita por primera vez el noctuido Allophyes corsica (Spuler, 1908) en el norte de la Península Ibérica, por lo que se amplía su distribución conocida. Los ejemplares estudiados han sido capturados en el Valle de Arán, en el Pi- rineo de Lérida, donde vuela a finales de octubre. PALABRAS CLAVE: Lepidoptera, Noctuidae, Psaphidinae, Allophyes corsica, Pirineos, Lérida, España. Allophyes corsica (Spuler, 1908) new species for the fauna of Spain (Lepidoptera: Noctuidae, Psaphidinae) Abstract The first record of the noctuid Allophyes corsica (Spuler, 1908) is reported from the Iberian Peninsula, increa- sing its known distribution. -

BOLLETTINO DELLA SOCIETÀ ENTOMOLOGICA ITALIANA Non-Commercial Use Only
BOLL.ENTOMOL_150_2_cover.qxp_Layout 1 07/09/18 07:42 Pagina a Poste Italiane S.p.A. ISSN 0373-3491 Spedizione in Abbonamento Postale - 70% DCB Genova BOLLETTINO DELLA SOCIETÀ ENTOMOLOGICA only ITALIANA use Volume 150 Fascicolo II maggio-agosto 2018Non-commercial 31 agosto 2018 SOCIETÀ ENTOMOLOGICA ITALIANA via Brigata Liguria 9 Genova BOLL.ENTOMOL_150_2_cover.qxp_Layout 1 07/09/18 07:42 Pagina b SOCIETÀ ENTOMOLOGICA ITALIANA Sede di Genova, via Brigata Liguria, 9 presso il Museo Civico di Storia Naturale n Consiglio Direttivo 2018-2020 Presidente: Francesco Pennacchio Vice Presidente: Roberto Poggi Segretario: Davide Badano Amministratore/Tesoriere: Giulio Gardini Bibliotecario: Antonio Rey only Direttore delle Pubblicazioni: Pier Mauro Giachino Consiglieri: Alberto Alma, Alberto Ballerio,use Andrea Battisti, Marco A. Bologna, Achille Casale, Marco Dellacasa, Loris Galli, Gianfranco Liberti, Bruno Massa, Massimo Meregalli, Luciana Tavella, Stefano Zoia Revisori dei Conti: Enrico Gallo, Sergio Riese, Giuliano Lo Pinto Revisori dei Conti supplenti: Giovanni Tognon, Marco Terrile Non-commercial n Consulenti Editoriali PAOLO AUDISIO (Roma) - EMILIO BALLETTO (Torino) - MAURIZIO BIONDI (L’Aquila) - MARCO A. BOLOGNA (Roma) PIETRO BRANDMAYR (Cosenza) - ROMANO DALLAI (Siena) - MARCO DELLACASA (Calci, Pisa) - ERNST HEISS (Innsbruck) - MANFRED JÄCH (Wien) - FRANCO MASON (Verona) - LUIGI MASUTTI (Padova) - MASSIMO MEREGALLI (Torino) - ALESSANDRO MINELLI (Padova)- IGNACIO RIBERA (Barcelona) - JOSÉ M. SALGADO COSTAS (Leon) - VALERIO SBORDONI (Roma) - BARBARA KNOFLACH-THALER (Innsbruck) - STEFANO TURILLAZZI (Firenze) - ALBERTO ZILLI (Londra) - PETER ZWICK (Schlitz). ISSN 0373-3491 BOLLETTINO DELLA SOCIETÀ ENTOMOLOGICA ITALIANA only use Fondata nel 1869 - Eretta a Ente Morale con R. Decreto 28 Maggio 1936 Volume 150 Fascicolo II maggio-agosto 2018Non-commercial 31 agosto 2018 REGISTRATO PRESSO IL TRIBUNALE DI GENOVA AL N. -
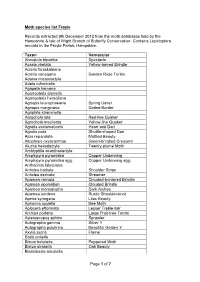
Page 1 of 7 Moth Species List Froyle Records
Moth species list Froyle Records extracted 9th December 2012 from the moth databases held by the Hampshire & Isle of Wight Branch of Butterfly Conservation. Contains Lepidoptera records in the Froyle Parish, Hampshire. Taxon Vernacular Abrostola tripartita Spectacle Acasis viretata Yellow-barred Brindle Acleris forsskaleana Acleris variegana Garden Rose Tortrix Adaina microdactyla Adela rufimitrella Agapeta hamana Agonopterix arenella Agonopterix heracliana Agriopis leucophaearia Spring Usher Agriopis marginaria Dotted Border Agriphila straminella Agrochola lota Red-line Quaker Agrochola macilenta Yellow-line Quaker Agrotis exclamationis Heart and Dart Agrotis puta Shuttle-shaped Dart Alcis repandata Mottled Beauty Allophyes oxyacanthae Green-brindled Crescent Alucita hexadactyla Twenty-plume Moth Amblyptilia acanthadactyla Amphipyra pyramidea Copper Underwing Amphipyra pyramidea agg. Copper Underwing agg. Anthophila fabriciana Anticlea badiata Shoulder Stripe Anticlea derivata Streamer Apamea crenata Clouded-bordered Brindle Apamea epomidion Clouded Brindle Apamea monoglypha Dark Arches Apamea sordens Rustic Shoulder-knot Apeira syringaria Lilac Beauty Aphomia sociella Bee Moth Aplocera efformata Lesser Treble-bar Archips podana Large Fruit-tree Tortrix Asteroscopus sphinx Sprawler Autographa gamma Silver Y Autographa pulchrina Beautiful Golden Y Axylia putris Flame Batia unitella Biston betularia Peppered Moth Biston strataria Oak Beauty Blastobasis adustella Page 1 of 7 Blastobasis lacticolella Cabera exanthemata Common Wave Cabera -

Download Download
UNIVERSITY THOUGHT doi:10.5937/univtho7-15336 Publication in Natural Sciences, Vol. 7, No. 2, 2017, pp. 1-27. Original Scientific Paper A CONTRIBUTION TO KNOWLEDGE OF THE BALKAN LEPIDOPTERA. SOME PYRALOIDEA (LEPIDOPTERA: CRAMBIDAE & PYRALIDAE) ENCOUNTERED RECENTLY IN SOUTHERN SERBIA, MONTENEGRO, THE REPUBLIC OF MACEDONIA AND ALBANIA COLIN W. PLANT1*, STOYAN BESHKOV2, PREDRAG JAKŠIĆ3, ANA NAHIRNIĆ2 114 West Road, Bishops Stortford, Hertfordshire, CM23 3QP, England 2National Museum of Natural History, Sofia, Bulgaria 3Faculty of Natural Science and Mathematics, University of Priština, Kosovska Mitrovica, Serbia ABSTRACT Pyraloidea (Lepidoptera: Crambidae & Pyralidae) were sampled in the territories of southern Serbia, Montenegro, the Former Yugoslav Republic of Macedonia and Albania on a total of 53 occasions during 2014, 2016 and 2017. A total of 173 species is reported here, comprising 97 Crambidae and 76 Pyralidae. Based upon published data, 29 species appear to be new to the fauna of Serbia, 5 species are new to the fauna of Macedonia and 37 are new to the fauna of Albania. The data are discussed. Keywords: Faunistics, Serbia, Montenegro, Republic of Macedonia, Albania, Pyraloidea, Pyralidae, Crambidae. of light trap. Some sites were visited on more than one occasion; INTRODUCTION others were sampled once only. Pyraloidea (Lepidoptera: Crambidae and Pyralidae) have As a by-product of this work, all remaining material from been examined in detail in the neighbouring territory of the the traps was returned to Sofia where Dr Boyan Zlatkov was Republic of Bulgaria and the results have been published by one given the opportunity to extract the Tortricoidea. The remaining of us (Plant, 2016). That work presented data for the 386 species material was retained and sent by post to England after the end of and 3 additional subspecies known from that country. -

U.S. EPA, Pesticide Product Label, JAVELIN WG, 03/25/2008
. '. .3 - d-S-J-oog- . UNITED S(=~ES ENVIRONMENTAL PROTECTION AG('~CY Christine A. Dively MAR 25 2008 Director of Regulatory Affairs Certis USA 9145 Guilford Road, Suite 175 Columbia, MD 21046 Dear Ms. Dively: Subject: Javelin WG (EPA Reg. No. 70051-66) Label submitted for Bt reregistration !' . As part of the reregistration ofBt microbial pesticides, the Agency has issued a stamped approved label for each reregistered product. Part of the labeling that was required for reregistrationwas an Environmental Hazards statement containing language to protect endangered or threatened Lepidoptera. After finalizing reregistration of the subject product, the Agency was made aware that registrants were given an erroneous version of the Environmental Hazard statement to include on product labels. .The correct statement should read as follows: "This product must not be applied aerially within 1/4 mile of any habitats of endangered or threatened Lepidoptera. No manual application can be made within 300 feet of any threatened or endangered Lepidoptera." . In light of this error, EPA has re-stamped the label forthe subject product with the corrected Environmental Hazards text. A copy of the label is enclosed for yoUr records. If you have any questions, please contact Alan Reynolds of my staff at (703) 605-0515 (e-mail: [email protected]). Sincerely, Sheryl Reilly, Ph.D., Branch Chief Microbial Pesticides Branch (7511P) Biopesticides and Pollution Prevention Division Enclosure CONCURRENCES' SYMBOL .. '/ S"lj () '. SURNAME ~ ··4····· ·lJ..~·: .....................................