Comamonas Testosteroni an Unusual Pathogen Shreekant Tiwari, Monalisah Nanda1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comamonas: Relationship to Aquaspirillum Aquaticum, E
INTERNATIONALJOURNAL OF SYSTEMATICBACTERIOLOGY, July 1991, p. 427-444 Vol. 41, No. 3 0020-7713/91/030427- 18$02 .OO/O Copyright 0 1991, International Union of Microbiological Societies Polyphasic Taxonomic Study of the Emended Genus Comamonas: Relationship to Aquaspirillum aquaticum, E. Falsen Group 10, and Other Clinical Isolates A. WILLEMS,l B. POT,l E. FALSEN,2 P. VANDAMME,' M. GILLIS,l* K. KERSTERS,l AND J. DE LEY' Laboratorium voor Microbiologie en Microbiele Genetica, Rijksuniversiteit, B-9000 Ghent, Belgium, and Culture Collection, Department of Clinical Bacteriology, University of Goteborg, S-413 46 Goteborg, Sweden2 We used DNA-rRNA hybridization, DNA base composition, polyacrylamide gel electrophoresis of whole-cell proteins, DNA-DNA hybridization, numerical analysis of phenotypic features, and immunotyping to study the taxonomy of the genus Comamonas. The relationships of this genus to Aquaspirillum aquaticum and a group of clinical isolates (E. Falsen group 10 [EF lo]) were studied. Our DNA and rRNA hybridization results indicate that the genus Comamonas consists of at least the following five genotypic groups: (i) Comamonas acidovoruns, (ii) Comamonas fesfosferoni,(iii) Comamonas ferrigena, (iv) A. aquaticum and a number of EF 10 strains, and (v) other EF 10 strains, several unnamed clinical isolates, and some misnamed strains of Pseudomonas alcaligenes and Pseudomonas pseudoalcaligenes subsp. pseudoalcaligenes. The existence of these five groups was confirmed by the results of immunotyping and protein gel electrophoresis. A numerical analysis of morpho- logical, auxanographic, and biochemical data for the same organisms revealed the existence of three large phena. Two of these phena (C. acidovorans and C. tesfosferoni)correspond to two of the genotypic groups. -

Breast Milk Microbiota: a Review of the Factors That Influence Composition
Published in "Journal of Infection 81(1): 17–47, 2020" which should be cited to refer to this work. ✩ Breast milk microbiota: A review of the factors that influence composition ∗ Petra Zimmermann a,b,c,d, , Nigel Curtis b,c,d a Department of Paediatrics, Fribourg Hospital HFR and Faculty of Science and Medicine, University of Fribourg, Switzerland b Department of Paediatrics, The University of Melbourne, Parkville, Australia c Infectious Diseases Research Group, Murdoch Children’s Research Institute, Parkville, Australia d Infectious Diseases Unit, The Royal Children’s Hospital Melbourne, Parkville, Australia s u m m a r y Breastfeeding is associated with considerable health benefits for infants. Aside from essential nutrients, immune cells and bioactive components, breast milk also contains a diverse range of microbes, which are important for maintaining mammary and infant health. In this review, we summarise studies that have Keywords: investigated the composition of the breast milk microbiota and factors that might influence it. Microbiome We identified 44 studies investigating 3105 breast milk samples from 2655 women. Several studies Diversity reported that the bacterial diversity is higher in breast milk than infant or maternal faeces. The maxi- Delivery mum number of each bacterial taxonomic level detected per study was 58 phyla, 133 classes, 263 orders, Caesarean 596 families, 590 genera, 1300 species and 3563 operational taxonomic units. Furthermore, fungal, ar- GBS chaeal, eukaryotic and viral DNA was also detected. The most frequently found genera were Staphylococ- Antibiotics cus, Streptococcus Lactobacillus, Pseudomonas, Bifidobacterium, Corynebacterium, Enterococcus, Acinetobacter, BMI Rothia, Cutibacterium, Veillonella and Bacteroides. There was some evidence that gestational age, delivery Probiotics mode, biological sex, parity, intrapartum antibiotics, lactation stage, diet, BMI, composition of breast milk, Smoking Diet HIV infection, geographic location and collection/feeding method influence the composition of the breast milk microbiota. -

Comamonas Kerstersii Bacteremia in a Patient with Acute Perforated
® Clinical Case Report Medicine OPEN Comamonas kerstersii bacteremia in a patient with acute perforated appendicitis A rare case report ∗ Yun-heng Zhou, PhDa, Hong-xia Ma, MDb, Zhao-yang Dong, PhDc, Mei-hua Shen, PhDd, Abstract Rationale: Comamonas species are rarely associated with human infections. Recent reports found that Comamonas kerstersii was associated with severe diseases such as abdominal infection and bacteremia. However, C. kerstersii maybe be confused with Comamonas testosteroni using the automatic bacterial identification systems currently available. Patient concerns: A 31-year-old man who had onset of left upper abdominal pain developed clinical manifestations of right lower abdominal pain and classic migration of pain at the temperature of 39°C. The positive strain of aerobic and anaerobic bottles of blood cultures was identified. Diagnoses: The patient was diagnosed as acute peritonitis and perforated appendix with abdominal abscess. Interventions: The bacterium was identified by routine methods, MALDI-TOF-MS and PCR amplification of the 16S rRNA. The patient was treated with exploratory laparotomy, appendectomy, tube drainage, and prescribing antibiotic treatment. Outcomes: The patients were discharged with complete recovery. The organisms were confirmed as C. kerstersii by MALDI-TOF- MS and a combination of the other results. Lessons: Our findings suggest that C. kerstersii infection occurs most often in association with perforated appendix and bacteremia. We presume that C. kerstersii is an opportunistic pathogen or commensal with the digestive tract and appendix bacteria. Abbreviations: C. kerstersii = Comamonas kerstersii, MALDI-TOF-MS = matrix-assisted laser desorption ionization–time of flight mass spectrometry, MIC = minimum inhibitory concentration, PCR = polymerase chain reaction. -

Delftia Rhizosphaerae Sp. Nov. Isolated from the Rhizosphere of Cistus Ladanifer
TAXONOMIC DESCRIPTION Carro et al., Int J Syst Evol Microbiol 2017;67:1957–1960 DOI 10.1099/ijsem.0.001892 Delftia rhizosphaerae sp. nov. isolated from the rhizosphere of Cistus ladanifer Lorena Carro,1† Rebeca Mulas,2 Raquel Pastor-Bueis,2 Daniel Blanco,3 Arsenio Terrón,4 Fernando Gonzalez-Andr es, 2 Alvaro Peix5,6 and Encarna Velazquez 1,6,* Abstract A bacterial strain, designated RA6T, was isolated from the rhizosphere of Cistus ladanifer. Phylogenetic analyses based on 16S rRNA gene sequence placed the isolate into the genus Delftia within a cluster encompassing the type strains of Delftia lacustris, Delftia tsuruhatensis, Delftia acidovorans and Delftia litopenaei, which presented greater than 97 % sequence similarity with respect to strain RA6T. DNA–DNA hybridization studies showed average relatedness ranging from of 11 to 18 % between these species of the genus Delftia and strain RA6T. Catalase and oxidase were positive. Casein was hydrolysed but gelatin and starch were not. Ubiquinone 8 was the major respiratory quinone detected in strain RA6T together with low amounts of ubiquinones 7 and 9. The major fatty acids were those from summed feature 3 (C16 : 1!7c/C16 : 1 !6c) and C16 : 0. The predominant polar lipids were diphosphatidylglycerol, phosphatidylglycerol and phosphatidylethanolamine. Phylogenetic, chemotaxonomic and phenotypic analyses showed that strain RA6T should be considered as a representative of a novel species of genus Delftia, for which the name Delftia rhizosphaerae sp. nov. is proposed. The type strain is RA6T (=LMG 29737T= CECT 9171T). The genus Delftia comprises Gram-stain-negative, non- The strain was grown on nutrient agar (NA; Sigma) for 48 h sporulating, strictly aerobic rods, motile by polar or bipolar at 22 C to check for motility by phase-contrast microscopy flagella. -

Sparus Aurata) and Sea Bass (Dicentrarchus Labrax)
Gut bacterial communities in geographically distant populations of farmed sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) Eleni Nikouli1, Alexandra Meziti1, Efthimia Antonopoulou2, Eleni Mente1, Konstantinos Ar. Kormas1* 1 Department of Ichthyology and Aquatic Environment, School of Agricultural Sciences, University of Thessaly, 384 46 Volos, Greece 2 Laboratory of Animal Physiology, Department of Zoology, School of Biology, Aristotle University of Thessaloniki, 541 24 Thessaloniki, Greece * Corresponding author; Tel.: +30-242-109-3082, Fax: +30-242109-3157, E-mail: [email protected], [email protected] Supplementary material 1 Table S1. Body weight of the Sparus aurata and Dicentrarchus labrax individuals used in this study. Chania Chios Igoumenitsa Yaltra Atalanti Sample Body weight S. aurata D. labrax S. aurata D. labrax S. aurata D. labrax S. aurata D. labrax S. aurata D. labrax (g) 1 359 378 558 420 433 448 481 346 260 785 2 355 294 579 442 493 556 516 397 240 340 3 376 275 468 554 450 464 540 415 440 500 4 392 395 530 460 440 483 492 493 365 860 5 420 362 483 479 542 492 406 995 6 521 505 506 461 Mean 380.40 340.80 523.17 476.67 471.60 487.75 504.50 419.67 326.25 696.00 SEs 11.89 23.76 17.36 19.56 20.46 23.85 8.68 21.00 46.79 120.29 2 Table S2. Ingredients of the diets used at the time of sampling. Ingredient Sparus aurata Dicentrarchus labrax (6 mm; 350-450 g)** (6 mm; 450-800 g)** Crude proteins (%) 42 – 44 37 – 39 Crude lipids (%) 19 – 21 20 – 22 Nitrogen free extract (NFE) (%) 20 – 26 19 – 25 Crude cellulose (%) 1 – 3 2 – 4 Ash (%) 5.8 – 7.8 6.2 – 8.2 Total P (%) 0.7 – 0.9 0.8 – 1.0 Gross energy (MJ/Kg) 21.5 – 23.5 20.6 – 22.6 Classical digestible energy* (MJ/Kg) 19.5 18.9 Added vitamin D3 (I.U./Kg) 500 500 Added vitamin E (I.U./Kg) 180 100 Added vitamin C (I.U./Kg) 250 100 Feeding rate (%), i.e. -

Delftia Sp. LCW, a Strain Isolated from a Constructed Wetland Shows Novel Properties for Dimethylphenol Isomers Degradation Mónica A
Vásquez-Piñeros et al. BMC Microbiology (2018) 18:108 https://doi.org/10.1186/s12866-018-1255-z RESEARCHARTICLE Open Access Delftia sp. LCW, a strain isolated from a constructed wetland shows novel properties for dimethylphenol isomers degradation Mónica A. Vásquez-Piñeros1, Paula M. Martínez-Lavanchy1,2, Nico Jehmlich3, Dietmar H. Pieper4, Carlos A. Rincón1, Hauke Harms5, Howard Junca6 and Hermann J. Heipieper1* Abstract Background: Dimethylphenols (DMP) are toxic compounds with high environmental mobility in water and one of the main constituents of effluents from petro- and carbochemical industry. Over the last few decades, the use of constructed wetlands (CW) has been extended from domestic to industrial wastewater treatments, including petro-carbochemical effluents. In these systems, the main role during the transformation and mineralization of organic pollutants is played by microorganisms. Therefore, understanding the bacterial degradation processes of isolated strains from CWs is an important approach to further improvements of biodegradation processes in these treatment systems. Results: In this study, bacterial isolation from a pilot scale constructed wetland fed with phenols led to the identification of Delftia sp. LCW as a DMP degrading strain. The strain was able to use the o-xylenols 3,4-DMP and 2,3-DMP as sole carbon and energy sources. In addition, 3,4-DMP provided as a co-substrate had an effect on the transformation of other four DMP isomers. Based on the detection of the genes, proteins, and the inferred phylogenetic relationships of the detected genes with other reported functional proteins, we found that the phenol hydroxylase of Delftia sp. LCW is induced by 3,4-DMP and it is responsible for the first oxidation of the aromatic ring of 3,4-, 2,3-, 2,4-, 2,5- and 3,5-DMP. -

Unveiling Bacterial Interactions Through Multidimensional Scaling and Dynamics Modeling Received: 06 May 2015 Pedro Dorado-Morales1, Cristina Vilanova1, Carlos P
www.nature.com/scientificreports OPEN Unveiling Bacterial Interactions through Multidimensional Scaling and Dynamics Modeling Received: 06 May 2015 Pedro Dorado-Morales1, Cristina Vilanova1, Carlos P. Garay3, Jose Manuel Martí3 Accepted: 17 November 2015 & Manuel Porcar1,2 Published: 16 December 2015 We propose a new strategy to identify and visualize bacterial consortia by conducting replicated culturing of environmental samples coupled with high-throughput sequencing and multidimensional scaling analysis, followed by identification of bacteria-bacteria correlations and interactions. We conducted a proof of concept assay with pine-tree resin-based media in ten replicates, which allowed detecting and visualizing dynamical bacterial associations in the form of statistically significant and yet biologically relevant bacterial consortia. There is a growing interest on disentangling the complexity of microbial interactions in order to both optimize reactions performed by natural consortia and to pave the way towards the development of synthetic consor- tia with improved biotechnological properties1,2. Despite the enormous amount of metagenomic data on both natural and artificial microbial ecosystems, bacterial consortia are not necessarily deduced from those data. In fact, the flexibility of the bacterial interactions, the lack of replicated assays and/or biases associated with differ- ent DNA isolation technologies and taxonomic bioinformatics tools hamper the clear identification of bacterial consortia. We propose here a holistic approach aiming at identifying bacterial interactions in laboratory-selected microbial complex cultures. The method requires multi-replicated taxonomic data on independent subcultures, and high-throughput sequencing-based taxonomic data. From this data matrix, randomness of replicates can be verified, linear correlations can be visualized and interactions can emerge from statistical correlations. -

Physiological and Genomic Features of Highly Alkaliphilic Hydrogen-Utilizing Betaproteobacteria from a Continental Serpentinizing Site
ARTICLE Received 17 Dec 2013 | Accepted 16 Apr 2014 | Published 21 May 2014 DOI: 10.1038/ncomms4900 OPEN Physiological and genomic features of highly alkaliphilic hydrogen-utilizing Betaproteobacteria from a continental serpentinizing site Shino Suzuki1, J. Gijs Kuenen2,3, Kira Schipper1,3, Suzanne van der Velde2,3, Shun’ichi Ishii1, Angela Wu1, Dimitry Y. Sorokin3,4, Aaron Tenney1, XianYing Meng5, Penny L. Morrill6, Yoichi Kamagata5, Gerard Muyzer3,7 & Kenneth H. Nealson1,2 Serpentinization, or the aqueous alteration of ultramafic rocks, results in challenging environments for life in continental sites due to the combination of extremely high pH, low salinity and lack of obvious electron acceptors and carbon sources. Nevertheless, certain Betaproteobacteria have been frequently observed in such environments. Here we describe physiological and genomic features of three related Betaproteobacterial strains isolated from highly alkaline (pH 11.6) serpentinizing springs at The Cedars, California. All three strains are obligate alkaliphiles with an optimum for growth at pH 11 and are capable of autotrophic growth with hydrogen, calcium carbonate and oxygen. The three strains exhibit differences, however, regarding the utilization of organic carbon and electron acceptors. Their global distribution and physiological, genomic and transcriptomic characteristics indicate that the strains are adapted to the alkaline and calcium-rich environments represented by the terrestrial serpentinizing ecosystems. We propose placing these strains in a new genus ‘Serpentinomonas’. 1 J. Craig Venter Institute, 4120 Torrey Pines Road, La Jolla, California 92037, USA. 2 University of Southern California, 835 W. 37th St. SHS 560, Los Angeles, California 90089, USA. 3 Delft University of Technology, Julianalaan 67, Delft, 2628BC, The Netherlands. -
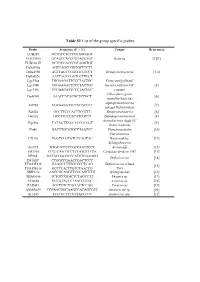
Table S1 List of the Group Specific Probes
Table S1 List of the group specific probes. Probe Sequence (5’ – 3’) Target References EUB338 GCTGCCTCCCGTAGGAGT EUB338 II GCAGCCACCCGTAGGTGT Bacteria [1][2] EUB338 III GCTGCCACCCGTAGGTGT Delta495a AGTTAGCCGGTGCTTCTT Delta495b AGTTAGCCGGCGCTTCCT Deltaproteobacteria [3,4] Delta495c AATTAGCCGGTGCTTCCT Lgc354a TGGAAGATTCCCTACTGC Firmicutes (Gram+ Lgc354b CGGAAGATTCCCTACTGC bacteria with low GC [5] Lgc354c CCGAAGATTCCCTACTGC content) Chloroflexi (green Gnsb941 AAACCACACGCTCCGCT [6] nonsulfur bacteria) Alphaproteobacteria Alf968 GGTAAGGTTCTGCGCGTT [7] (except Rickettsiales) Bet42a GCCTTCCCACTTCGTTT Betaproteobacteria [8] Gam2a GCCTTCCCACATCGTTT Gammaproteobacteria [8] Actinobacteria (high GC Hgc69a TATAGTTACCACCGCCGT [9] Gram+ bacteria) Pla46 GACTTGCATGCCTAATCC Planctomycetales [10] Flavobacteria, Cf319a TGGTCCGTGTCTCAGTAC Bacteroidetes, [11] Sphingobacteria Arc915 GTGCTCCCCCGCCAATTCCT Archaea [12] TM7905 CCGTCAATTCCTTTATGTTTTA Candidate division TM7 [13] DF988* GATACGACGCCCATGTCAAGGG Defluvicoccus [14] DF1020* CCGGCCGAACCGACTCCC TFO-DF218 GAAGCCTTTGCCCCTCAG Defluvicoccus related [15] TFO-DF618 GCCTCACTTGTCTAACCG TFO SBR9-1a AAGCGCAAGTTCCCAGGTTG Sphingomonas [16] THAU646 TCTGCCGTACTCTAGCCTT Thauera sp. [17] AZO644 GCCGTACTCTAGCCGTGC Azoarcus sp. [18] PAR651 ACCTCTCTCGAACTCCAG Paracoccus [19] AMAR839 CCGAACGGCAAGCCACAGCGTC Amaricoccus sp. [20] ACI145 TTTCGCTTCGTTATCCCC Acidovorax spp. [21] Table S2 Primers used in PCR and Sequencing. Primers Sequence (5’ – 3’) PCR 27f AGAGTTTGATCMTGGCTCAG 1492r TACGGYTACCTTGTTACGACTT T7f TAATACGACTCACTATAGGG -

Insights from Comamonas Testosteroni
International Journal of Molecular Sciences Review Chemotaxis Towards Aromatic Compounds: Insights from Comamonas testosteroni Yun-Hao Wang 1,2, Zhou Huang 1 and Shuang-Jiang Liu 1,2,* 1 State Key Laboratory of Microbial Resources and Environmental Microbiology Research Center, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China; [email protected] (Y.-H.W.); [email protected] (Z.H.) 2 University of Chinese Academy of Sciences, Beijing 101408, China * Correspondence: [email protected]; Tel.: +86-10-64807423 Received: 21 April 2019; Accepted: 30 May 2019; Published: 1 June 2019 Abstract: Chemotaxis is an important physiological adaptation that allows many motile bacteria to orientate themselves for better niche adaptation. Chemotaxis is best understood in Escherichia coli. Other representative bacteria, such as Rhodobacter sphaeroides, Pseudomonas species, Helicobacter pylori, and Bacillus subtilis, also have been deeply studied and systemically summarized. These bacteria belong to α-, γ-, "-Proteobacteria, or Firmicutes. However, β-Proteobacteria, of which many members have been identified as holding chemotactic pathways, lack a summary of chemotaxis. Comamonas testosteroni, belonging to β-Proteobacteria, grows with and chemotactically responds to a range of aromatic compounds. This paper summarizes the latest research on chemotaxis towards aromatic compounds, mainly from investigations of C. testosteroni and other Comamonas species. Keywords: Comamonas testosteroni; chemoreceptor; chemotaxis; aromatic compounds 1. Introduction Aromatic compounds have one or more aromatic rings containing resonance bonds, which make them chemically inert due to charge distribution over the whole skeleton of the aromatic ring. It has been estimated that about 25% of photosynthetic products from plants are deposited as lignin, which is one of the most abundant aromatic compounds on earth [1,2]. -

Identification of Pseudomonas Species and Other Non-Glucose Fermenters
UK Standards for Microbiology Investigations Identification of Pseudomonas species and other Non- Glucose Fermenters Issued by the Standards Unit, Microbiology Services, PHE Bacteriology – Identification | ID 17 | Issue no: 3 | Issue date: 13.04.15 | Page: 1 of 41 © Crown copyright 2015 Identification of Pseudomonas species and other Non-Glucose Fermenters Acknowledgments UK Standards for Microbiology Investigations (SMIs) are developed under the auspices of Public Health England (PHE) working in partnership with the National Health Service (NHS), Public Health Wales and with the professional organisations whose logos are displayed below and listed on the website https://www.gov.uk/uk- standards-for-microbiology-investigations-smi-quality-and-consistency-in-clinical- laboratories. SMIs are developed, reviewed and revised by various working groups which are overseen by a steering committee (see https://www.gov.uk/government/groups/standards-for-microbiology-investigations- steering-committee). The contributions of many individuals in clinical, specialist and reference laboratories who have provided information and comments during the development of this document are acknowledged. We are grateful to the Medical Editors for editing the medical content. For further information please contact us at: Standards Unit Microbiology Services Public Health England 61 Colindale Avenue London NW9 5EQ E-mail: [email protected] Website: https://www.gov.uk/uk-standards-for-microbiology-investigations-smi-quality- and-consistency-in-clinical-laboratories -

Burkholderiales Participating in Pentachlorophenol Biodegradation in Iron-Reducing
Electronic Supplementary Material (ESI) for Environmental Science: Processes & Impacts. This journal is © The Royal Society of Chemistry 2015 Burkholderiales participating in pentachlorophenol biodegradation in iron-reducing paddy soil as identified by stable isotope probing Hui Tong a,b,c,1, Min Hu b,1, Fangbai Li *b, Manjia Chen b, Yahui Lv b,d a Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou 510640, PR China b Guangdong Key Laboratory of Agricultural Environment Pollution Integrated Control, Guangdong Institute of Eco-Environmental and Soil Sciences, Guangzhou 510650, PR China c Graduate University of Chinese Academy of Sciences, Beijing 100049, PR China d School of Chemical and Environmental Engineering, Shanghai Institute of Technology, Shanghai 201418, PR China * Corresponding author. Tel.: +86 20 87024721; fax: +86 20 87024123. E-mail address: [email protected] (F.B., Li). 1These authors contributed equally to this work. Number of pages: 5 Number of figures: 1 Number of tables: 2 Figure S1 The relative abundance of T-RFs in the 13C-DNA heavy fractions. The solid line is the model-fitted relative abundance using the pseudo-first-order kinetic model. Table S1. Phylogenetic affiliation of two (A and B) 16S rRNA clone in PCP degrading microcosms. A 12C-Heavy fraction of clones Phylum Class Order Family Genus 8 Proteobacteria Gammaproteobacteria Enterobacteriales Enterobacteriaceae Escherichia/Shigella 7 Proteobacteria Betaproteobacteria Unclassified 4 Planctomycetes Planctomycetacia Planctomycetales Planctomycetaceae