Front Matter
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lecture Notes on Human Anatomy. Part One, Fourth Edition. PUB DATE Sep 89 NOTE 79P.; for Related Documents, See SE 051 219-221
DOCUMENT RESUME ED 315 320 SE 051 218 AUTHOR Conrey, Kathleen TITLE Lecture Notes on Human Anatomy. Part One, Fourth Edition. PUB DATE Sep 89 NOTE 79p.; For related documents, see SE 051 219-221. Black and white illustrations will not reproduce clearly. AVAILABLE FROM Aramaki Design and Publications, 12077 Jefferson Blvd., Culver City, CA 90506 ($7.75). PUB TYPE Guides - Classroom Use - Materials (For Learner) (051) EDRS PRICE MF01 Plus Postage. PC Not Available from EDRS. DESCRIPTORS *Anatomy; *Biological Sciences; *College Science; Higher Education; *Human Body; *Lecture Method; Science Education; Secondary Education; Secondary School Science; Teaching Guides; Teaching Methods ABSTRACT During the process of studying the specific course content of human anatomy, students are being educated to expand their vocabulary, deal successfully with complex tasks, anduse a specific way of thinking. This is the first volume in a set of notes which are designed to accompany a lecture series in human anatomy. This volume Includes discussions of anatomical planes and positions, body cavities, and architecture; studies of the skeleton including bones and joints; studies of the musculature of the body; and studiesof the nervous system including the central, autonomic, motor and sensory systems. (CW) *****1.**k07********Y*******t1.****+***********,****A*******r****** % Reproductions supplied by EDRS are the best that can be made from the original document. **************************************************************A**t***** "PERMISSION TO REPRODUCE -

Specification of Optic Nerve Oligodendrocyte Precursors by Retinal Ganglion Cell Axons
The Journal of Neuroscience, July 19, 2006 • 26(29):7619–7628 • 7619 Cellular/Molecular Specification of Optic Nerve Oligodendrocyte Precursors by Retinal Ganglion Cell Axons Limin Gao and Robert H. Miller Department of Neurosciences, Case Western Reserve University, Cleveland, Ohio 44106 Cell fate commitment in the developing CNS frequently depends on localized cell–cell interactions. In the avian visual system the optic nerve oligodendrocytes are derived from founder cells located at the floor of the third ventricle. Here we show that the induction of these founder cells is directly dependent on signaling from the retinal ganglion cell (RGC) axons. The appearance of oligodendrocyte precursor cells (OPCs) correlates with the projection of RGC axons, and early eye removal dramatically reduces the number of OPCs. In vitro signaling from retinal neurites induces OPCs in responsive tissue. Retinal axon induction of OPCs is dependent on sonic hedgehog (Shh) andneuregulinsignaling,andtheinhibitionofeithersignalreducesOPCinductioninvivoandinvitro.ThedependenceofOPCsonretinal axonal cues appears to be a common phenomenon, because ocular retardation (orJ) mice lacking optic nerve have dramatically reduced OPCs in the midline of the third ventricle. Key words: oligodendrocyte precursors; optic nerve; axon induction; sonic hedgehog; neuregulin; retinal ganglion cells Introduction contributes to the specification of ventral midline cells (Dale et al., During development the oligodendrocytes, the myelinating cells 1997); however, OPCs arise later -

Early Neuronal Processes Interact with Glia to Establish a Scaffold For
bioRxiv preprint doi: https://doi.org/10.1101/754416; this version posted August 31, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Early neuronal processes interact with glia to establish a scaffold for orderly innervation of the cochlea Running Title: Glia and early cochlear wiring N. R. Druckenbrod1, E. B. Hale, O. O. Olukoya, W. E. Shatzer, and L.V. Goodrich* Department of Neurobiology, Harvard Medical School, Boston, MA, 02115 1Current address: Decibel Therapeutics, Boston, Ma, 02215 *correspondence should be addressed to [email protected] Keywords: neuron-glia interactions, axon guidance, spiral ganglion neuron, cochlea Authors’ contributions: This study was conceived of and designed by NRD and LVG. NRD, EBH, OOO, and WES performed experiments and analyzed results. LVG analyzed results and wrote the manuscript. bioRxiv preprint doi: https://doi.org/10.1101/754416; this version posted August 31, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Summary: Although the basic principles of axon guidance are well established, it remains unclear how axons navigate with high fidelity through the complex cellular terrains that are encountered in vivo. To learn more about the cellular strategies underlying axon guidance in vivo, we analyzed the developing cochlea, where spiral ganglion neurons extend processes through a heterogeneous cellular environment to form tonotopically ordered connections with hair cells. -

Vocabulario De Morfoloxía, Anatomía E Citoloxía Veterinaria
Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) Servizo de Normalización Lingüística Universidade de Santiago de Compostela COLECCIÓN VOCABULARIOS TEMÁTICOS N.º 4 SERVIZO DE NORMALIZACIÓN LINGÜÍSTICA Vocabulario de Morfoloxía, anatomía e citoloxía veterinaria (galego-español-inglés) 2008 UNIVERSIDADE DE SANTIAGO DE COMPOSTELA VOCABULARIO de morfoloxía, anatomía e citoloxía veterinaria : (galego-español- inglés) / coordinador Xusto A. Rodríguez Río, Servizo de Normalización Lingüística ; autores Matilde Lombardero Fernández ... [et al.]. – Santiago de Compostela : Universidade de Santiago de Compostela, Servizo de Publicacións e Intercambio Científico, 2008. – 369 p. ; 21 cm. – (Vocabularios temáticos ; 4). - D.L. C 2458-2008. – ISBN 978-84-9887-018-3 1.Medicina �������������������������������������������������������������������������veterinaria-Diccionarios�������������������������������������������������. 2.Galego (Lingua)-Glosarios, vocabularios, etc. políglotas. I.Lombardero Fernández, Matilde. II.Rodríguez Rio, Xusto A. coord. III. Universidade de Santiago de Compostela. Servizo de Normalización Lingüística, coord. IV.Universidade de Santiago de Compostela. Servizo de Publicacións e Intercambio Científico, ed. V.Serie. 591.4(038)=699=60=20 Coordinador Xusto A. Rodríguez Río (Área de Terminoloxía. Servizo de Normalización Lingüística. Universidade de Santiago de Compostela) Autoras/res Matilde Lombardero Fernández (doutora en Veterinaria e profesora do Departamento de Anatomía e Produción Animal. -

The Sympathetic and the Parasympathetic Nervous System
The sympathetic and the parasympathetic nervous system Zsuzsanna Tóth, PhD Institute of Anatomy, Histology and Embryology Semmelweis University The role of the autonomic nervous system Claude Bernard • „milieu intérieur” concept; every organism lives in its internal environment that is constant and independent form the external environment Walter Bradford Cannon homeostasis; • an extension of the “milieu interieur” concept • consistence in an open system requires mechanisms that act to maintain that consistency • steady-state conditions require that any tendency toward change automatically meets with factors that resist that change • regulating systems that determine the homeostatic state : o autonomic nervous system ( sympathetic, parasympathetic, enteral) o endocrine system General structure of the autonomic nervous system craniosacral thoracolumbar Anatomy Neurotransmittersof the gut autonomic nervous system. symp. gangl pregangl. fiber pregangl. postgangl. fiber fiber (PoR) PoR enteral ganglion PoR PoR smooth muscle smooth muscle Kuratani S Development 2009;136:1585-1589 Sympathetic activation: Fight or flight reaction • energy mobilization • preparation for escape, or fight vasoconstriction • generalized Parasympathetic activation: adrenal • energy saving and restoring • „rest and digest” system • more localized vasoconstriction Paravertebral ganglia and the sympathetic chains pars cervicalis superius ganglion medium cervicale stellatum pars vertebrae • from the base of the skull to the caudal end thoracalis thoracalis of the sacrum • paravertebral ganglia (ganglia trunci sympathici) • rami interganglionares pars vertebrae • the two chains fuses at the ganglion impar abdominalis lumbalis sacrum pars pelvina foramen sacralia anteriora ganglion impar Anatomy of the cervical part of the sympathetic trunk superior cervical ganglion • behind the seath of the carotid, fusiform ggl. cervicale superius • IML T1-3 vegetative motoneurons- preganglionic fibers truncus symp. -
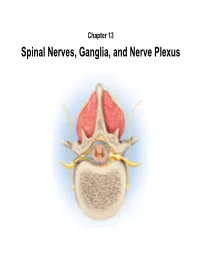
Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves
Chapter 13 Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves Posterior Spinous process of vertebra Posterior root Deep muscles of back Posterior ramus Spinal cord Transverse process of vertebra Posterior root ganglion Spinal nerve Anterior ramus Meningeal branch Communicating rami Anterior root Vertebral body Sympathetic ganglion Anterior General Anatomy of Nerves and Ganglia • Spinal cord communicates with the rest of the body by way of spinal nerves • nerve = a cordlike organ composed of numerous nerve fibers (axons) bound together by connective tissue – mixed nerves contain both afferent (sensory) and efferent (motor) fibers – composed of thousands of fibers carrying currents in opposite directions Anatomy of a Nerve Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Epineurium Perineurium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endoneurium Nerve Rootlets fiber Posterior root Fascicle Posterior root ganglion Anterior Blood root vessels Spinal nerve (b) Copyright by R.G. Kessel and R.H. Kardon, Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy, 1979, W.H. Freeman, All rights reserved Blood vessels Fascicle Epineurium Perineurium Unmyelinated nerve fibers Myelinated nerve fibers (a) Endoneurium Myelin General Anatomy of Nerves and Ganglia • nerves of peripheral nervous system are ensheathed in Schwann cells – forms neurilemma and often a myelin sheath around the axon – external to neurilemma, each fiber is surrounded by -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

The Anatomic Basis of Vertebrogenic Pain and the Autonomic Syndrome Associated with Lumbar Disk Extrusion
219 The Anatomic Basis of Vertebrogenic Pain and the Autonomic Syndrome Associated with Lumbar Disk Extrusion 1 2 John R. Jinkins • Extruded lumbar intervertebral disks traditionally have been classified as posterior or Anthony R. Whittemore 1 central in location. A retrospective review of 250 MR imaging examinations of the lumbar William G. Bradley1 spine that used mid- and high-field imagers revealed 145 positive studies, which included a significant number of extrusions extending anteriorly. With the lateral margin of the neural foramen/pedicle as the boundary, 29.2% of peripheral disk extrusions were anterior and 56.4% were posterior. In addition, a prevalence of 14.4% was found for central disk extrusions, in which there was a rupture of disk material into or through the vertebral body itself. The clinical state of neurogenic spinal radiculopathy accom panying posterior disk extrusion has been well defined; however, uncomplicated anterior and central disk extrusions also may be associated with a definite clinical syndrome. The vertebrogenic symptom complex includes (1) local and referred pain and (2) autonomic reflex dysfunction within the lumbosacral zones of Head. Generalized alter ations in viscerosomatic tone potentially may also be observed. The anatomic basis for the mediation of clinical signs and symptoms generated within the disk and paradiskal structures rests with afferent sensory fibers from two primary sources: (1) posterolateral neural branches emanating from the ventral ramus of the somatic spinal root and (2) neural rami projecting directly to the paravertebral autonomic neural plexus. Thus, conscious perception and unconscious effects originating in the vertebral column, although complex, have definite pathways represented in this dual peripheral innervation associated with intimately related andfor parallel central ramifications. -

The Autonomic Nervous System of Selachians. by John Z
The Autonomic Nervous System of Selachians. By John Z. Young, B.A. With 28 Text-figures. CONTENTS. I. INTRODUCTION ......... 571 II. MATERIAL AND METHODS 572 III. ANATOMY AND HISTOLOGY OF THE SYMPATHETIC SYSTEM . 575 1. Nature of the Rami Communicantes .... 575 2. Anterior Eami Communicantes and Sympathetic Ganglia 581 3. Anatomy of the Sympathetic System in the Trunk . 581 4. Sympathetic System and Suprarenals in the Kidney Kegion 586 5. Sympathetic System in the Tail ..... 589 6. Autonomic Fibres in Spinal Dorsal Roots . 593 7. Cytology of the Sympathetic Ganglia .... 593 8. Relation of the Sympathetic Cells to the Suprarenal Tissue 598 9. Post-Branchial Plexus 600 IV. INNERVATION OF THE VISCERA 603 1. Nerves of the Alimentary Canal ..... 603 2. Innervation of the Urinogenital System . 609 3. Cardiac Nerves 610 V. CRANIAL AUTONOMIC SYSTEM 610 1. Autonomic Fibres in Branchial Nerves . 610 2. Profundus and Ciliary Nerves ..... 614 VI. PHYLOGENETIC HISTORY OF THE AUTONOMIC NERVOUS SYSTEM 617 VII. SUMMARY. 621 VIII. BIBLIOGRAPHY 623 I. INTRODUCTION. THE only complete account of the sympathetic nervous system of Selachians is that of Chevrel published in 1887. Since that date several papers have appeared dealing with special points of structure or function, such as those of Bottazzi (1902), Muller and Liljestrand (1918), and Lutz (1981), on the innerva- tion of the viscera; of Diamare (1901) on the histology; and of NO. 300 o o 572 JOHN Z. YOUNG Hoffmann (1900), Miiller (1920), and others, on the development. No attempt has yet been made to investigate the autonomic nervous system of these fish from the general standpoint intro- duced by Langley (1921) and Gaskell (1915); this the present study attempts to do. -

Differences in Neural Stem Cell Identity and Differentiation Capacity Drive Divergent Regenerative Outcomes in Lizards and Salamanders
Differences in neural stem cell identity and differentiation capacity drive divergent regenerative outcomes in lizards and salamanders Aaron X. Suna,b,c, Ricardo Londonoa, Megan L. Hudnalla, Rocky S. Tuana,c, and Thomas P. Lozitoa,1 aCenter for Cellular and Molecular Engineering, Department of Orthopaedic Surgery, University of Pittsburgh School of Medicine, Pittsburgh, PA 15219; bMedical Scientist Training Program, University of Pittsburgh School of Medicine, Pittsburgh, PA 15213; and cDepartment of Bioengineering, University of Pittsburgh Swanson School of Engineering, Pittsburgh, PA 15213 Edited by Robb Krumlauf, Stowers Institute for Medical Research, Kansas City, MO, and approved July 24, 2018 (received for review March 2, 2018) While lizards and salamanders both exhibit the ability to re- lizard tail regenerate (20), and the key to understanding this generate amputated tails, the outcomes achieved by each are unique arrangement of tissues is in identifying the patterning markedly different. Salamanders, such as Ambystoma mexicanum, signals involved. regenerate nearly identical copies of original tails. Regenerated lizard Both lizards and salamanders follow similar mechanisms of tails, however, exhibit important morphological differences compared tail development during embryonic development. The embryonic with originals. Some of these differences concern dorsoventral pat- spinal cord and surrounding structures are formed and patterned terning of regenerated skeletal and spinal cord tissues; regenerated by the neural tube (21, 22). The neural tube exhibits distinct + salamander tail tissues exhibit dorsoventral patterning, while re- domains: roof plate (characterized by expression of Pax7 , BMP- + + + grown lizard tissues do not. Additionally, regenerated lizard tails lack 2 , and Sox10 among others), lateral domain (Pax6 ), and floor + + characteristically roof plate-associated structures, such as dorsal root plate (Shh , FoxA2 ). -

Reaction of Ependymal Cells to Spinal Cord Injury: a Potential Role for Oncostatin Pathway and Microglial Cells
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.12.428106; this version posted February 13, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Reaction of ependymal cells to spinal cord injury: a potential role for oncostatin pathway and microglial cells *1 *1 *1 2 2 1 R. Chevreau , H Ghazale , C Ripoll , C Chalfouh , Q Delarue , A.L. Hemonnot , H 1 3 4 5 *2 *1 Hirbec , S Wahane , F Perrin , H Noristani , N Guerout , JP Hugnot *equal contribution, listed in alphabetic order 1 Université de Montpellier, INSERM CNRS IGF, France 2 Université de Rouen, France 3 Departments of Neurobiology and Neurosurgery, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles CA 90095-1763, USA. 4 University of Montpellier, INSERM MMDN France 5 Shriners Hospitals Pediatric Research Center, NY, USA Abstract Ependymal cells with stem cell properties reside in the adult spinal cord around the central canal. They rapidly activate and proliferate after spinal cord injury, constituting a source of new cells. They produce neurons and glial cells in lower vertebrates but they mainly generate glial cells in mammals. The mechanisms underlying their activation and their glial-biased differentiation in mammals remain ill-defined. This represents an obstacle to control these cells. We addressed this issue using RNA profiling of ependymal cells before and after injury. We found that these cells activate STAT3 and ERK/MAPK signaling during injury and downregulate cilia-associated genes and FOXJ1, a central transcription factor in ciliogenesis. -

Canine Dorsal Root Ganglia Satellite Glial Cells Represent an Exceptional Cell Population with Astrocytic and Oligodendrocytic P
www.nature.com/scientificreports OPEN Canine dorsal root ganglia satellite glial cells represent an exceptional cell population with astrocytic and Received: 17 August 2017 Accepted: 6 October 2017 oligodendrocytic properties Published: xx xx xxxx W. Tongtako1,2, A. Lehmbecker1, Y. Wang1,2, K. Hahn1,2, W. Baumgärtner1,2 & I. Gerhauser 1 Dogs can be used as a translational animal model to close the gap between basic discoveries in rodents and clinical trials in humans. The present study compared the species-specifc properties of satellite glial cells (SGCs) of canine and murine dorsal root ganglia (DRG) in situ and in vitro using light microscopy, electron microscopy, and immunostainings. The in situ expression of CNPase, GFAP, and glutamine synthetase (GS) has also been investigated in simian SGCs. In situ, most canine SGCs (>80%) expressed the neural progenitor cell markers nestin and Sox2. CNPase and GFAP were found in most canine and simian but not murine SGCs. GS was detected in 94% of simian and 71% of murine SGCs, whereas only 44% of canine SGCs expressed GS. In vitro, most canine (>84%) and murine (>96%) SGCs expressed CNPase, whereas GFAP expression was diferentially afected by culture conditions and varied between 10% and 40%. However, GFAP expression was induced by bone morphogenetic protein 4 in SGCs of both species. Interestingly, canine SGCs also stimulated neurite formation of DRG neurons. These fndings indicate that SGCs represent an exceptional, intermediate glial cell population with phenotypical characteristics of oligodendrocytes and astrocytes and might possess intrinsic regenerative capabilities in vivo. Since the discovery of glial cells over a century ago, substantial progress has been made in understanding the origin, development, and function of the diferent types of glial cells in the central nervous system (CNS) and peripheral nervous system (PNS)1.