Early Neuronal Processes Interact with Glia to Establish a Scaffold For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Specification of Optic Nerve Oligodendrocyte Precursors by Retinal Ganglion Cell Axons
The Journal of Neuroscience, July 19, 2006 • 26(29):7619–7628 • 7619 Cellular/Molecular Specification of Optic Nerve Oligodendrocyte Precursors by Retinal Ganglion Cell Axons Limin Gao and Robert H. Miller Department of Neurosciences, Case Western Reserve University, Cleveland, Ohio 44106 Cell fate commitment in the developing CNS frequently depends on localized cell–cell interactions. In the avian visual system the optic nerve oligodendrocytes are derived from founder cells located at the floor of the third ventricle. Here we show that the induction of these founder cells is directly dependent on signaling from the retinal ganglion cell (RGC) axons. The appearance of oligodendrocyte precursor cells (OPCs) correlates with the projection of RGC axons, and early eye removal dramatically reduces the number of OPCs. In vitro signaling from retinal neurites induces OPCs in responsive tissue. Retinal axon induction of OPCs is dependent on sonic hedgehog (Shh) andneuregulinsignaling,andtheinhibitionofeithersignalreducesOPCinductioninvivoandinvitro.ThedependenceofOPCsonretinal axonal cues appears to be a common phenomenon, because ocular retardation (orJ) mice lacking optic nerve have dramatically reduced OPCs in the midline of the third ventricle. Key words: oligodendrocyte precursors; optic nerve; axon induction; sonic hedgehog; neuregulin; retinal ganglion cells Introduction contributes to the specification of ventral midline cells (Dale et al., During development the oligodendrocytes, the myelinating cells 1997); however, OPCs arise later -
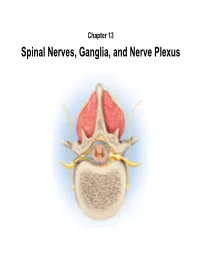
Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves
Chapter 13 Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves Posterior Spinous process of vertebra Posterior root Deep muscles of back Posterior ramus Spinal cord Transverse process of vertebra Posterior root ganglion Spinal nerve Anterior ramus Meningeal branch Communicating rami Anterior root Vertebral body Sympathetic ganglion Anterior General Anatomy of Nerves and Ganglia • Spinal cord communicates with the rest of the body by way of spinal nerves • nerve = a cordlike organ composed of numerous nerve fibers (axons) bound together by connective tissue – mixed nerves contain both afferent (sensory) and efferent (motor) fibers – composed of thousands of fibers carrying currents in opposite directions Anatomy of a Nerve Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Epineurium Perineurium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endoneurium Nerve Rootlets fiber Posterior root Fascicle Posterior root ganglion Anterior Blood root vessels Spinal nerve (b) Copyright by R.G. Kessel and R.H. Kardon, Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy, 1979, W.H. Freeman, All rights reserved Blood vessels Fascicle Epineurium Perineurium Unmyelinated nerve fibers Myelinated nerve fibers (a) Endoneurium Myelin General Anatomy of Nerves and Ganglia • nerves of peripheral nervous system are ensheathed in Schwann cells – forms neurilemma and often a myelin sheath around the axon – external to neurilemma, each fiber is surrounded by -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Digital Neuroanatomy
DIGITAL NEUROANATOMY LM NEUROHISTOLOGY George R. Leichnetz, Ph.D. Professor, Department of Anatomy & Neurobiology Virginia Commonwealth University 2004 Press the Å and Æ keys on your keyboard to navigate through this lecture There are three morphological types of neurons in the nervous system: unipolar, bipolar, and multipolar. While all three types can be found in the PNS, the CNS only contains multipolar neurons. CNS multipolar neurons vary greatly in morphology: eg. spinal cord motor neurons, pyramidal cells of cerebral cortex, Purkinje cells of cerebellar cortex. The supportive cells of the CNS are neuroglia: astrocytes, oligodendrocytes, and microglia. Oligodendrocytes myelinate CNS axons, while Schwann cells myelinate PNS axons. Protoplasmic astrocytes predominate in CNS gray matter, while fibrous astrocytes predominate in CNS white matter. The ventral horn of the spinal cord Lumbar Spinal Cord contains multipolar motor neurons. Dorsal White Horn Matter Gray Matter Ventral Kluver- Horn Barrera Stain (LFB & CV) Cell bodies of motor neurons Dendrite Gray Matter Dendrite Perineuronal H & E oligodendrocyte Stain Nucleus, nucleolus Abundant Nissl substance Dorsal root Dorsal root ganglion Ventral root Gray Matter: contains White Matter: cell bodies of neurons contains x-sections of myelinated axons Multipolar motor neurons Silver Stain of the ventral horn Large multipolar motor neurons of the spinal cord ventral horn: see nucleus, nucleolus, abundant Nissl substance, multiple tapering processes (dendrites). The axon hillock (origin of axon from cell body) lacks Nissl. Nucleus Axon hillock dendrites Nucleolus Nissl substance (RER) Kluver-Barrera Stain (Luxol Fast Blue and Cresyl Violet) Thoracic Spinal Cord The nucleus dorsalis of Clarke, located in the base of the dorsal horn of the thoracic spinal cord, contains multipolar neurons whose nucleus is eccentric and Nissl around the periphery of the cell body (as if it were chromatolytic). -

Gathering at the Nodes
RESEARCH HIGHLIGHTS GLIA Gathering at the nodes Saltatory conduction — the process by which that are found at the nodes of Ranvier, where action potentials propagate along myelinated they interact with Na+ channels. When the nerves — depends on the fact that voltage- authors either disrupted the localization of gated Na+ channels form clusters at the nodes gliomedin by using a soluble fusion protein of Ranvier, between sections of the myelin that contained the extracellular domain of sheath. New findings from Eshed et al. show neurofascin, or used RNA interference to that Schwann cells produce a protein called suppress the expression of gliomedin, the gliomedin, and that this is responsible for characteristic clustering of Na+ channels at the clustering of these channels. the nodes of Ranvier did not occur. The formation of the nodes of Ranvier is Aggregation of the domain of gliomedin specified by the myelinating cells, not the that binds neurofascin and NrCAM on the axons, and an important component of this surface of purified neurons also caused process in the peripheral nervous system the clustering of neurofascin, Na+ channels is the extension of microvilli by Schwann and other nodal proteins. These findings cells. These microvilli contact the axons at support a model in which gliomedin on the nodes, and it is here that the Schwann References and links Schwann cell microvilli binds to neurofascin ORIGINAL RESEARCH PAPER Eshed, Y. et al. Gliomedin cells express the newly discovered protein and NrCAM on axons, causing them to mediates Schwann cell–axon interaction and the molecular gliomedin. cluster at the nodes of Ranvier, and leading assembly of the nodes of Ranvier. -

Histochemical Evidence for Microglia-Like Macrophages in the Rat Trigeminal Ganglion
J. Anat. (1993) 183, pp. 475-481, with 5 figures Printed in Great Britain 475 Histochemical evidence for microglia-like macrophages in the rat trigeminal ganglion J. A. GLENN, J. B. SONCEAU, H. J. WYNDER* AND W. E. THOMAS Department of Oral Biology, College of Dentistry, Ohio State University, Columbus, Ohio, USA (Accepted 8 April 1993) ABSTRACT Of the 4 major cell types in CNS parenchyma, microglia appear to serve the unique functional role of tissue macrophages. The distribution of equivalent cells in the PNS is unclear. Recently, the B4 isolectin of Griffonia simplicifolia was shown to bind selectively to microglia as well as to other macrophages under specific conditions. In the present study, this lectin was used to assess the existence of macrophages in the rat trigeminal ganglion. Vibratome sections of fixed ganglia were incubated with horseradish peroxidase (HRP)-conjugated isolectin, an HRP reaction subsequently performed, and sections processed for histology and viewed by light microscopy. Staining activity was found to be localised to a population of cells throughout the ganglion. These cells possessed small oval somata and several thin crenated processes, an appearance typical of ramified microglia. Stained cells also exhibited a regular, evenly spaced tissue distribution similar to CNS microglia. Finally, similar cells were also labelled by thiamine pyrophosphatase histochemistry, a cellular marker for CNS microglia/macrophages. It was concluded that there are microglia-like macrophages in the trigeminal ganglion and that these cells may function in immune reactions. responding alterations in cellular morphology INTRODUCTION (Thomas, 1992). As a unique component of neural tissue, microglial In possibly the best studied example of microglial cells have been supported by recent studies as intrinsic function as macrophages, involving the facial nucleus macrophages and thus may function in immune of the CNS, it has been demonstrated that these cells reactions in this tissue. -

THE Mandffiular GANGLION - a NEW PERIPHERAL GANGLION of the LOCUST
J. exp. Biol. 148, 313-324 (1990) 313 Primed in Great Britain © The Company of Biologists Limited 1990 THE MANDffiULAR GANGLION - A NEW PERIPHERAL GANGLION OF THE LOCUST BY PETER BRAUNIG Institut fiir Zoologie, Technische Universitdt Milnchen, Lichtenbergstrafle 4, D-8046 Garching, Federal Republic of Germany Accepted 22 August 1989 Summary Paired peripheral ganglia within the locust mandibular segment are described. Each mandibular ganglion contains the cell bodies of 22-25 neurones. Four of these are sensory neurones which innervate the receptor strand of one of the mandibular proprioceptors. The other neurones connect the suboesophageal ganglion with the tritocerebral lobes of the brain, and with the first ganglion of the stomatogastric nervous system, the frontal ganglion. Introduction In addition to the chain of segmental ganglia of the central nervous system (CNS), insects possess a stomatogastric nervous system which innervates the foregut. It consists of the unpaired frontal and hypocerebral ganglia and the paired paraventricular ganglia. It is connected with the central nervous system (CNS) via the frontal connectives, which link the frontal ganglion to both tritocerebral lobes of the supraoesophageal ganglion, or brain (see Fig. 1). Nerve cells making connections with the frontal ganglion have been found chiefly in the brain, but there are also a few in the suboesophageal ganglion (Aubele and Klemm, 1977; Gundel and Penzlin, 1978; Kirby etal. 1984). Thus, both head ganglia appear to participate in the control of the stomatogastric nervous system. In the course of a study of the peripheral nervous system of the locust suboesophageal ganglion (Braunig, 1987), peculiar structures were found in association with one of the major branches of the mandibular nerve. -

Oligodendrocytes and CNS Myelin Are Nonpermissive Substrates for Neurite Growth and Fibroblast Spreading in Vi&O
The Journal of Neuroscience, July 1988, 8(7): 2381-2393 Oligodendrocytes and CNS Myelin Are Nonpermissive Substrates for Neurite Growth and Fibroblast Spreading in vi&o Martin E. Schwab and Pica Caroni Brain Research Institute of the University of Zurich, CH-8029 Zurich, Switzerland To study the interaction of neurons with CNS glial cells, (Tello, 1911; Ramon y Cajal, 1928; Benfey and Aguayo, 1982; dissociated sympathetic or sensory ganglion cells or fetal Richardson et al., 1984; So and Aguayo, 1985). These studies retinal cells were plated onto cultures of dissociated optic assigneda crucial role to the microenvironment of the growing nerve glial cells of young rats. Whereas astrocytes favored fibers, whereby peripheral nerve tissue should allow, support, neuron adhesion and neurite outgrowth, oligodendrocytes or provoke neurite regeneration. The involvement of neuro- differed markedly in their properties as neuronal substrates. trophic and neurotropic factors-produced by Schwann cellsbut Immature (O,+, A,B,+, GalCm) oligodendrocytes were fre- not by CNS glia-was suggested60 years ago by Ramon y Cajal quently contacted by neurons and neurites. In contrast, dif- (1928). In fact, a marked increase in the production of neuro- ferentiated oligodendrocytes (O,+, A$-, GalC+) represented trophic factors and cell adhesionmolecules by Schwann cells in a nonpermissive substrate for neuronal adhesion and neurite responseto denervation has recently been observed (Richardson growth. When neuroblastoma cells or 3T3 fibroblasts were and Ebendal, 1982; Longo et al., 1984; Abrahamson et al., 1986; plated into optic nerve glial cultures, the same differences Daniloff et al., 1986). However, neurotrophic factors are also were observed; differentiated oligodendrocytes were non- presentin developing and adult CNS, and increasedneurotroph- permissive for cell adhesion, neurite growth, or fibroblast ic activities were found at sites of CNS lesions (Barde et al., spreading. -

The Spinal Cord and Spinal Nerves
14 The Nervous System: The Spinal Cord and Spinal Nerves PowerPoint® Lecture Presentations prepared by Steven Bassett Southeast Community College Lincoln, Nebraska © 2012 Pearson Education, Inc. Introduction • The Central Nervous System (CNS) consists of: • The spinal cord • Integrates and processes information • Can function with the brain • Can function independently of the brain • The brain • Integrates and processes information • Can function with the spinal cord • Can function independently of the spinal cord © 2012 Pearson Education, Inc. Gross Anatomy of the Spinal Cord • Features of the Spinal Cord • 45 cm in length • Passes through the foramen magnum • Extends from the brain to L1 • Consists of: • Cervical region • Thoracic region • Lumbar region • Sacral region • Coccygeal region © 2012 Pearson Education, Inc. Gross Anatomy of the Spinal Cord • Features of the Spinal Cord • Consists of (continued): • Cervical enlargement • Lumbosacral enlargement • Conus medullaris • Cauda equina • Filum terminale: becomes a component of the coccygeal ligament • Posterior and anterior median sulci © 2012 Pearson Education, Inc. Figure 14.1a Gross Anatomy of the Spinal Cord C1 C2 Cervical spinal C3 nerves C4 C5 C 6 Cervical C 7 enlargement C8 T1 T2 T3 T4 T5 T6 T7 Thoracic T8 spinal Posterior nerves T9 median sulcus T10 Lumbosacral T11 enlargement T12 L Conus 1 medullaris L2 Lumbar L3 Inferior spinal tip of nerves spinal cord L4 Cauda equina L5 S1 Sacral spinal S nerves 2 S3 S4 S5 Coccygeal Filum terminale nerve (Co1) (in coccygeal ligament) Superficial anatomy and orientation of the adult spinal cord. The numbers to the left identify the spinal nerves and indicate where the nerve roots leave the vertebral canal. -

Activation of Ganglion Cells and Axon Bundles Using Epiretinal Electrical Stimulation
J Neurophysiol 118: 1457–1471, 2017. First published May 31, 2017; doi:10.1152/jn.00750.2016. RESEARCH ARTICLE Biology of Neuroengineering Interfaces Activation of ganglion cells and axon bundles using epiretinal electrical stimulation Lauren E. Grosberg,1 Karthik Ganesan,2 Georges A. Goetz,1 Sasidhar S. Madugula,1 Nandita Bhaskhar,2 Victoria Fan,1 Peter Li,3 Pawel Hottowy,4 Wladyslaw Dabrowski,4 Alexander Sher,5 Alan M. Litke,5 Subhasish Mitra,2 and E. J. Chichilnisky1 1Department of Neurosurgery and Hansen Experimental Physics Laboratory, Stanford University, Stanford, California; 2Departments of Electrical Engineering and Computer Science, Stanford University, Stanford, California; 3Systems Downloaded from Neurobiology Laboratories, Salk Institute for Biological Studies, La Jolla, California; 4Faculty of Physics and Applied Computer Science, AGH University of Science and Technology, Krakow, Poland; and 5Santa Cruz Institute for Particle Physics, University of California, Santa Cruz, Santa Cruz, California Submitted 19 September 2016; accepted in final form 30 May 2017 http://jn.physiology.org/ Grosberg LE, Ganesan K, Goetz GA, Madugula SS, Bhaskhar retinal electrophysiology; retinal prosthesis; brain-machine interface; N, Fan V, Li P, Hottowy P, Dabrowski W, Sher A, Litke AM, retinal ganglion cells; axon bundles; raphe Mitra S, Chichilnisky EJ. Activation of ganglion cells and axon bundles using epiretinal electrical stimulation. J Neurophysiol 118: 1457–1471, 2017. First published May 31, 2017; doi:10.1152/ RETINAL PROSTHESES are designed to restore partial visual func- jn.00750.2016.—Epiretinal prostheses for treating blindness activate tion in patients blinded by photoreceptor degeneration. These axon bundles, causing large, arc-shaped visual percepts that limit the devices operate by using electrode arrays to activate retinal quality of artificial vision. -

Nonneuronal Control of the Differential Distribution of Myelin Along Retinal Ganglion Cell Axons in the Mouse
Anatomy and Pathology Nonneuronal Control of the Differential Distribution of Myelin Along Retinal Ganglion Cell Axons in the Mouse Xiaoli Yang,*,1 Haidong Zou,†,1 Gila Jung,1 Gisbert Richard,1 Stephan J. Linke,1 Marius Ader,2 and Udo Bartsch1 1Department of Ophthalmology, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany 2Center for Regenerative Therapies Dresden, TU Dresden, Germany Correspondence: Udo Bartsch, De- PURPOSE. In most mammalian species, retinal ganglion cell (RGC) axons are myelinated in the partment of Ophthalmology, Univer- optic nerve, but remain nonmyelinated in the retinal nerve fiber layer and the most proximal sity Medical Centre Hamburg- (i.e., retina-near) region of the nerve. Here we analyzed whether RGCs are involved in the Eppendorf, Martinistr. 52, 20246 control of this characteristic distribution of oligodendrocytes and myelin in the primary visual Hamburg, Germany; pathway of mice. [email protected]. METHODS. Neurospheres were enriched in oligodendrocyte progenitor cells (OPCs) by a short- XY and HZ contributed equally to the work presented here and should term exposure to platelet-derived growth factor (PDGF) and grafted into the retina of young therefore be regarded as equivalent postnatal mice close to the optic disc. Immunohistochemistry was performed to study the authors. integration and differentiation of the grafted cells, and the formation of donor-derived myelin in the normally nonmyelinated retinal nerve fiber layer and intrabulbar and most proximal Current affiliation: *Department of retrobulbar region of the optic nerve. Ophthalmology, North Sichuan Medi- cal College, Nanchong Sichuan, China. RESULTS. Intraretinal transplantations of small-sized PDGF-treated neurospheres into young †Department of Ophthalmology, postnatal mice resulted in extensive integration of the grafted cells into host retinas. -

Harnessing Astrocytes and Müller Glial Cells in the Retina for Survival and Regeneration of Retinal Ganglion Cells
cells Review Harnessing Astrocytes and Müller Glial Cells in the Retina for Survival and Regeneration of Retinal Ganglion Cells Hyung-Suk Yoo, Ushananthini Shanmugalingam and Patrice D. Smith * Department of Neuroscience, Carleton University, Ottawa, ON K1S 5B6, Canada; [email protected] (H.-S.Y.); [email protected] (U.S.) * Correspondence: [email protected] Abstract: Astrocytes have been associated with the failure of axon regeneration in the central nervous system (CNS), as it undergoes reactive gliosis in response to damages to the CNS and functions as a chemical and physical barrier to axon regeneration. However, beneficial roles of astrocytes have been extensively studied in the spinal cord over the years, and a growing body of evidence now suggests that inducing astrocytes to become more growth-supportive can promote axon regeneration after spinal cord injury (SCI). In retina, astrocytes and Müller cells are known to undergo reactive gliosis after damage to retina and/or optic nerve and are hypothesized to be either detrimental or beneficial to survival and axon regeneration of retinal ganglion cells (RGCs). Whether they can be induced to become more growth-supportive after retinal and optic nerve injury has yet to be determined. In this review, we pinpoint the potential molecular pathways involved in the induction of growth-supportive astrocytes in the spinal cord and suggest that stimulating the activation of these pathways in the retina could represent a new therapeutic approach to promoting survival and axon regeneration of RGCs in retinal degenerative diseases. Citation: Yoo, H.-S.; Shanmugalingam, U.; Smith, P.D. Keywords: macroglia; astrocytes; Müller cells; optic nerve crush; retinal ganglion cells; spinal cord Harnessing Astrocytes and Müller injury; signal transducer and activator of transcription 3; epidermal growth factor Glial Cells in the Retina for Survival and Regeneration of Retinal Ganglion Cells.