Developing Dorsal Root Ganglion Neurons Require Trophic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Specification of Optic Nerve Oligodendrocyte Precursors by Retinal Ganglion Cell Axons
The Journal of Neuroscience, July 19, 2006 • 26(29):7619–7628 • 7619 Cellular/Molecular Specification of Optic Nerve Oligodendrocyte Precursors by Retinal Ganglion Cell Axons Limin Gao and Robert H. Miller Department of Neurosciences, Case Western Reserve University, Cleveland, Ohio 44106 Cell fate commitment in the developing CNS frequently depends on localized cell–cell interactions. In the avian visual system the optic nerve oligodendrocytes are derived from founder cells located at the floor of the third ventricle. Here we show that the induction of these founder cells is directly dependent on signaling from the retinal ganglion cell (RGC) axons. The appearance of oligodendrocyte precursor cells (OPCs) correlates with the projection of RGC axons, and early eye removal dramatically reduces the number of OPCs. In vitro signaling from retinal neurites induces OPCs in responsive tissue. Retinal axon induction of OPCs is dependent on sonic hedgehog (Shh) andneuregulinsignaling,andtheinhibitionofeithersignalreducesOPCinductioninvivoandinvitro.ThedependenceofOPCsonretinal axonal cues appears to be a common phenomenon, because ocular retardation (orJ) mice lacking optic nerve have dramatically reduced OPCs in the midline of the third ventricle. Key words: oligodendrocyte precursors; optic nerve; axon induction; sonic hedgehog; neuregulin; retinal ganglion cells Introduction contributes to the specification of ventral midline cells (Dale et al., During development the oligodendrocytes, the myelinating cells 1997); however, OPCs arise later -

Early Neuronal Processes Interact with Glia to Establish a Scaffold For
bioRxiv preprint doi: https://doi.org/10.1101/754416; this version posted August 31, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Early neuronal processes interact with glia to establish a scaffold for orderly innervation of the cochlea Running Title: Glia and early cochlear wiring N. R. Druckenbrod1, E. B. Hale, O. O. Olukoya, W. E. Shatzer, and L.V. Goodrich* Department of Neurobiology, Harvard Medical School, Boston, MA, 02115 1Current address: Decibel Therapeutics, Boston, Ma, 02215 *correspondence should be addressed to [email protected] Keywords: neuron-glia interactions, axon guidance, spiral ganglion neuron, cochlea Authors’ contributions: This study was conceived of and designed by NRD and LVG. NRD, EBH, OOO, and WES performed experiments and analyzed results. LVG analyzed results and wrote the manuscript. bioRxiv preprint doi: https://doi.org/10.1101/754416; this version posted August 31, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Summary: Although the basic principles of axon guidance are well established, it remains unclear how axons navigate with high fidelity through the complex cellular terrains that are encountered in vivo. To learn more about the cellular strategies underlying axon guidance in vivo, we analyzed the developing cochlea, where spiral ganglion neurons extend processes through a heterogeneous cellular environment to form tonotopically ordered connections with hair cells. -
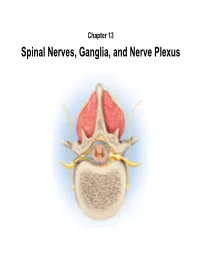
Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves
Chapter 13 Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves Posterior Spinous process of vertebra Posterior root Deep muscles of back Posterior ramus Spinal cord Transverse process of vertebra Posterior root ganglion Spinal nerve Anterior ramus Meningeal branch Communicating rami Anterior root Vertebral body Sympathetic ganglion Anterior General Anatomy of Nerves and Ganglia • Spinal cord communicates with the rest of the body by way of spinal nerves • nerve = a cordlike organ composed of numerous nerve fibers (axons) bound together by connective tissue – mixed nerves contain both afferent (sensory) and efferent (motor) fibers – composed of thousands of fibers carrying currents in opposite directions Anatomy of a Nerve Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Epineurium Perineurium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endoneurium Nerve Rootlets fiber Posterior root Fascicle Posterior root ganglion Anterior Blood root vessels Spinal nerve (b) Copyright by R.G. Kessel and R.H. Kardon, Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy, 1979, W.H. Freeman, All rights reserved Blood vessels Fascicle Epineurium Perineurium Unmyelinated nerve fibers Myelinated nerve fibers (a) Endoneurium Myelin General Anatomy of Nerves and Ganglia • nerves of peripheral nervous system are ensheathed in Schwann cells – forms neurilemma and often a myelin sheath around the axon – external to neurilemma, each fiber is surrounded by -

Spinal Cord Organization
Lecture 4 Spinal Cord Organization The spinal cord . Afferent tract • connects with spinal nerves, through afferent BRAIN neuron & efferent axons in spinal roots; reflex receptor interneuron • communicates with the brain, by means of cell ascending and descending pathways that body form tracts in spinal white matter; and white matter muscle • gives rise to spinal reflexes, pre-determined gray matter Efferent neuron by interneuronal circuits. Spinal Cord Section Gross anatomy of the spinal cord: The spinal cord is a cylinder of CNS. The spinal cord exhibits subtle cervical and lumbar (lumbosacral) enlargements produced by extra neurons in segments that innervate limbs. The region of spinal cord caudal to the lumbar enlargement is conus medullaris. Caudal to this, a terminal filament of (nonfunctional) glial tissue extends into the tail. terminal filament lumbar enlargement conus medullaris cervical enlargement A spinal cord segment = a portion of spinal cord that spinal ganglion gives rise to a pair (right & left) of spinal nerves. Each spinal dorsal nerve is attached to the spinal cord by means of dorsal and spinal ventral roots composed of rootlets. Spinal segments, spinal root (rootlets) nerve roots, and spinal nerves are all identified numerically by th region, e.g., 6 cervical (C6) spinal segment. ventral Sacral and caudal spinal roots (surrounding the conus root medullaris and terminal filament and streaming caudally to (rootlets) reach corresponding intervertebral foramina) collectively constitute the cauda equina. Both the spinal cord (CNS) and spinal roots (PNS) are enveloped by meninges within the vertebral canal. Spinal nerves (which are formed in intervertebral foramina) are covered by connective tissue (epineurium, perineurium, & endoneurium) rather than meninges. -

Digital Neuroanatomy
DIGITAL NEUROANATOMY LM NEUROHISTOLOGY George R. Leichnetz, Ph.D. Professor, Department of Anatomy & Neurobiology Virginia Commonwealth University 2004 Press the Å and Æ keys on your keyboard to navigate through this lecture There are three morphological types of neurons in the nervous system: unipolar, bipolar, and multipolar. While all three types can be found in the PNS, the CNS only contains multipolar neurons. CNS multipolar neurons vary greatly in morphology: eg. spinal cord motor neurons, pyramidal cells of cerebral cortex, Purkinje cells of cerebellar cortex. The supportive cells of the CNS are neuroglia: astrocytes, oligodendrocytes, and microglia. Oligodendrocytes myelinate CNS axons, while Schwann cells myelinate PNS axons. Protoplasmic astrocytes predominate in CNS gray matter, while fibrous astrocytes predominate in CNS white matter. The ventral horn of the spinal cord Lumbar Spinal Cord contains multipolar motor neurons. Dorsal White Horn Matter Gray Matter Ventral Kluver- Horn Barrera Stain (LFB & CV) Cell bodies of motor neurons Dendrite Gray Matter Dendrite Perineuronal H & E oligodendrocyte Stain Nucleus, nucleolus Abundant Nissl substance Dorsal root Dorsal root ganglion Ventral root Gray Matter: contains White Matter: cell bodies of neurons contains x-sections of myelinated axons Multipolar motor neurons Silver Stain of the ventral horn Large multipolar motor neurons of the spinal cord ventral horn: see nucleus, nucleolus, abundant Nissl substance, multiple tapering processes (dendrites). The axon hillock (origin of axon from cell body) lacks Nissl. Nucleus Axon hillock dendrites Nucleolus Nissl substance (RER) Kluver-Barrera Stain (Luxol Fast Blue and Cresyl Violet) Thoracic Spinal Cord The nucleus dorsalis of Clarke, located in the base of the dorsal horn of the thoracic spinal cord, contains multipolar neurons whose nucleus is eccentric and Nissl around the periphery of the cell body (as if it were chromatolytic). -

Canine Dorsal Root Ganglia Satellite Glial Cells Represent an Exceptional Cell Population with Astrocytic and Oligodendrocytic P
www.nature.com/scientificreports OPEN Canine dorsal root ganglia satellite glial cells represent an exceptional cell population with astrocytic and Received: 17 August 2017 Accepted: 6 October 2017 oligodendrocytic properties Published: xx xx xxxx W. Tongtako1,2, A. Lehmbecker1, Y. Wang1,2, K. Hahn1,2, W. Baumgärtner1,2 & I. Gerhauser 1 Dogs can be used as a translational animal model to close the gap between basic discoveries in rodents and clinical trials in humans. The present study compared the species-specifc properties of satellite glial cells (SGCs) of canine and murine dorsal root ganglia (DRG) in situ and in vitro using light microscopy, electron microscopy, and immunostainings. The in situ expression of CNPase, GFAP, and glutamine synthetase (GS) has also been investigated in simian SGCs. In situ, most canine SGCs (>80%) expressed the neural progenitor cell markers nestin and Sox2. CNPase and GFAP were found in most canine and simian but not murine SGCs. GS was detected in 94% of simian and 71% of murine SGCs, whereas only 44% of canine SGCs expressed GS. In vitro, most canine (>84%) and murine (>96%) SGCs expressed CNPase, whereas GFAP expression was diferentially afected by culture conditions and varied between 10% and 40%. However, GFAP expression was induced by bone morphogenetic protein 4 in SGCs of both species. Interestingly, canine SGCs also stimulated neurite formation of DRG neurons. These fndings indicate that SGCs represent an exceptional, intermediate glial cell population with phenotypical characteristics of oligodendrocytes and astrocytes and might possess intrinsic regenerative capabilities in vivo. Since the discovery of glial cells over a century ago, substantial progress has been made in understanding the origin, development, and function of the diferent types of glial cells in the central nervous system (CNS) and peripheral nervous system (PNS)1. -

Gathering at the Nodes
RESEARCH HIGHLIGHTS GLIA Gathering at the nodes Saltatory conduction — the process by which that are found at the nodes of Ranvier, where action potentials propagate along myelinated they interact with Na+ channels. When the nerves — depends on the fact that voltage- authors either disrupted the localization of gated Na+ channels form clusters at the nodes gliomedin by using a soluble fusion protein of Ranvier, between sections of the myelin that contained the extracellular domain of sheath. New findings from Eshed et al. show neurofascin, or used RNA interference to that Schwann cells produce a protein called suppress the expression of gliomedin, the gliomedin, and that this is responsible for characteristic clustering of Na+ channels at the clustering of these channels. the nodes of Ranvier did not occur. The formation of the nodes of Ranvier is Aggregation of the domain of gliomedin specified by the myelinating cells, not the that binds neurofascin and NrCAM on the axons, and an important component of this surface of purified neurons also caused process in the peripheral nervous system the clustering of neurofascin, Na+ channels is the extension of microvilli by Schwann and other nodal proteins. These findings cells. These microvilli contact the axons at support a model in which gliomedin on the nodes, and it is here that the Schwann References and links Schwann cell microvilli binds to neurofascin ORIGINAL RESEARCH PAPER Eshed, Y. et al. Gliomedin cells express the newly discovered protein and NrCAM on axons, causing them to mediates Schwann cell–axon interaction and the molecular gliomedin. cluster at the nodes of Ranvier, and leading assembly of the nodes of Ranvier. -

Gamma Motor Neurons Survive and Exacerbate Alpha Motor Neuron Degeneration In
Gamma motor neurons survive and exacerbate alpha PNAS PLUS motor neuron degeneration in ALS Melanie Lalancette-Heberta,b, Aarti Sharmaa,b, Alexander K. Lyashchenkoa,b, and Neil A. Shneidera,b,1 aCenter for Motor Neuron Biology and Disease, Columbia University, New York, NY 10032; and bDepartment of Neurology, Columbia University, New York, NY 10032 Edited by Rob Brownstone, University College London, London, United Kingdom, and accepted by Editorial Board Member Fred H. Gage October 27, 2016 (received for review April 4, 2016) The molecular and cellular basis of selective motor neuron (MN) the muscle spindle and control the sensitivity of spindle afferent vulnerability in amyotrophic lateral sclerosis (ALS) is not known. In discharge (15); beta (β) skeletofusimotor neurons innervate both genetically distinct mouse models of familial ALS expressing intra- and extrafusal muscle (16). In addition to morphological mutant superoxide dismutase-1 (SOD1), TAR DNA-binding protein differences, distinct muscle targets, and the absence of primary 43 (TDP-43), and fused in sarcoma (FUS), we demonstrate selective afferent (IA)inputsonγ-MNs, these functional MN subtypes also degeneration of alpha MNs (α-MNs) and complete sparing of differ in their trophic requirements, and γ-MNs express high levels gamma MNs (γ-MNs), which selectively innervate muscle spindles. of the glial cell line-derived neurotropic factor (GDNF) receptor Resistant γ-MNs are distinct from vulnerable α-MNs in that they Gfrα1 (17). γ-MNs are also molecularly distinguished by the ex- lack synaptic contacts from primary afferent (IA) fibers. Elimination pression of other selective markers including the transcription α of these synapses protects -MNs in the SOD1 mutant, implicating factor Err3 (18), Wnt7A (19), the serotonin receptor 1d (5-ht1d) this excitatory input in MN degeneration. -

Dorsal Root Injury—A Model for Exploring Pathophysiology and Therapeutic Strategies in Spinal Cord Injury
cells Review Dorsal Root Injury—A Model for Exploring Pathophysiology and Therapeutic Strategies in Spinal Cord Injury Håkan Aldskogius * and Elena N. Kozlova Laboratory of Regenertive Neurobiology, Biomedical Center, Department of Neuroscience, Uppsala University, 75124 Uppsala, Sweden; [email protected] * Correspondence: [email protected] Abstract: Unraveling the cellular and molecular mechanisms of spinal cord injury is fundamental for our possibility to develop successful therapeutic approaches. These approaches need to address the issues of the emergence of a non-permissive environment for axonal growth in the spinal cord, in combination with a failure of injured neurons to mount an effective regeneration program. Experimental in vivo models are of critical importance for exploring the potential clinical relevance of mechanistic findings and therapeutic innovations. However, the highly complex organization of the spinal cord, comprising multiple types of neurons, which form local neural networks, as well as short and long-ranging ascending or descending pathways, complicates detailed dissection of mechanistic processes, as well as identification/verification of therapeutic targets. Inducing different types of dorsal root injury at specific proximo-distal locations provide opportunities to distinguish key components underlying spinal cord regeneration failure. Crushing or cutting the dorsal root allows detailed analysis of the regeneration program of the sensory neurons, as well as of the glial response at the dorsal root-spinal cord interface without direct trauma to the spinal cord. At the same time, a lesion at this interface creates a localized injury of the spinal cord itself, but with an initial Citation: Aldskogius, H.; Kozlova, neuronal injury affecting only the axons of dorsal root ganglion neurons, and still a glial cell response E.N. -

Histochemical Evidence for Microglia-Like Macrophages in the Rat Trigeminal Ganglion
J. Anat. (1993) 183, pp. 475-481, with 5 figures Printed in Great Britain 475 Histochemical evidence for microglia-like macrophages in the rat trigeminal ganglion J. A. GLENN, J. B. SONCEAU, H. J. WYNDER* AND W. E. THOMAS Department of Oral Biology, College of Dentistry, Ohio State University, Columbus, Ohio, USA (Accepted 8 April 1993) ABSTRACT Of the 4 major cell types in CNS parenchyma, microglia appear to serve the unique functional role of tissue macrophages. The distribution of equivalent cells in the PNS is unclear. Recently, the B4 isolectin of Griffonia simplicifolia was shown to bind selectively to microglia as well as to other macrophages under specific conditions. In the present study, this lectin was used to assess the existence of macrophages in the rat trigeminal ganglion. Vibratome sections of fixed ganglia were incubated with horseradish peroxidase (HRP)-conjugated isolectin, an HRP reaction subsequently performed, and sections processed for histology and viewed by light microscopy. Staining activity was found to be localised to a population of cells throughout the ganglion. These cells possessed small oval somata and several thin crenated processes, an appearance typical of ramified microglia. Stained cells also exhibited a regular, evenly spaced tissue distribution similar to CNS microglia. Finally, similar cells were also labelled by thiamine pyrophosphatase histochemistry, a cellular marker for CNS microglia/macrophages. It was concluded that there are microglia-like macrophages in the trigeminal ganglion and that these cells may function in immune reactions. responding alterations in cellular morphology INTRODUCTION (Thomas, 1992). As a unique component of neural tissue, microglial In possibly the best studied example of microglial cells have been supported by recent studies as intrinsic function as macrophages, involving the facial nucleus macrophages and thus may function in immune of the CNS, it has been demonstrated that these cells reactions in this tissue. -

THE Mandffiular GANGLION - a NEW PERIPHERAL GANGLION of the LOCUST
J. exp. Biol. 148, 313-324 (1990) 313 Primed in Great Britain © The Company of Biologists Limited 1990 THE MANDffiULAR GANGLION - A NEW PERIPHERAL GANGLION OF THE LOCUST BY PETER BRAUNIG Institut fiir Zoologie, Technische Universitdt Milnchen, Lichtenbergstrafle 4, D-8046 Garching, Federal Republic of Germany Accepted 22 August 1989 Summary Paired peripheral ganglia within the locust mandibular segment are described. Each mandibular ganglion contains the cell bodies of 22-25 neurones. Four of these are sensory neurones which innervate the receptor strand of one of the mandibular proprioceptors. The other neurones connect the suboesophageal ganglion with the tritocerebral lobes of the brain, and with the first ganglion of the stomatogastric nervous system, the frontal ganglion. Introduction In addition to the chain of segmental ganglia of the central nervous system (CNS), insects possess a stomatogastric nervous system which innervates the foregut. It consists of the unpaired frontal and hypocerebral ganglia and the paired paraventricular ganglia. It is connected with the central nervous system (CNS) via the frontal connectives, which link the frontal ganglion to both tritocerebral lobes of the supraoesophageal ganglion, or brain (see Fig. 1). Nerve cells making connections with the frontal ganglion have been found chiefly in the brain, but there are also a few in the suboesophageal ganglion (Aubele and Klemm, 1977; Gundel and Penzlin, 1978; Kirby etal. 1984). Thus, both head ganglia appear to participate in the control of the stomatogastric nervous system. In the course of a study of the peripheral nervous system of the locust suboesophageal ganglion (Braunig, 1987), peculiar structures were found in association with one of the major branches of the mandibular nerve. -

Oligodendrocytes and CNS Myelin Are Nonpermissive Substrates for Neurite Growth and Fibroblast Spreading in Vi&O
The Journal of Neuroscience, July 1988, 8(7): 2381-2393 Oligodendrocytes and CNS Myelin Are Nonpermissive Substrates for Neurite Growth and Fibroblast Spreading in vi&o Martin E. Schwab and Pica Caroni Brain Research Institute of the University of Zurich, CH-8029 Zurich, Switzerland To study the interaction of neurons with CNS glial cells, (Tello, 1911; Ramon y Cajal, 1928; Benfey and Aguayo, 1982; dissociated sympathetic or sensory ganglion cells or fetal Richardson et al., 1984; So and Aguayo, 1985). These studies retinal cells were plated onto cultures of dissociated optic assigneda crucial role to the microenvironment of the growing nerve glial cells of young rats. Whereas astrocytes favored fibers, whereby peripheral nerve tissue should allow, support, neuron adhesion and neurite outgrowth, oligodendrocytes or provoke neurite regeneration. The involvement of neuro- differed markedly in their properties as neuronal substrates. trophic and neurotropic factors-produced by Schwann cellsbut Immature (O,+, A,B,+, GalCm) oligodendrocytes were fre- not by CNS glia-was suggested60 years ago by Ramon y Cajal quently contacted by neurons and neurites. In contrast, dif- (1928). In fact, a marked increase in the production of neuro- ferentiated oligodendrocytes (O,+, A$-, GalC+) represented trophic factors and cell adhesionmolecules by Schwann cells in a nonpermissive substrate for neuronal adhesion and neurite responseto denervation has recently been observed (Richardson growth. When neuroblastoma cells or 3T3 fibroblasts were and Ebendal, 1982; Longo et al., 1984; Abrahamson et al., 1986; plated into optic nerve glial cultures, the same differences Daniloff et al., 1986). However, neurotrophic factors are also were observed; differentiated oligodendrocytes were non- presentin developing and adult CNS, and increasedneurotroph- permissive for cell adhesion, neurite growth, or fibroblast ic activities were found at sites of CNS lesions (Barde et al., spreading.