Sarepta Therapeutics
Top View
- Antibody Testing and Gene Therapy
- 08/06/2016 Provider Subsystem Healthcare and Family Services Run Time: 00:10:55 Report Id 2794D052 Page: 01
- The Bottom 99
- Sarepta Therapeutics, Inc. (Nasdaqgs: SRPT) Updating Coverage
- Open-Label Evaluation of Eteplirsen in Patients with Duchenne Muscular Dystrophy Amenable to Exon 51 Skipping: PROMOVI Trial Craig Mcdonald,1 Perry Shieh,2 Hoda Z
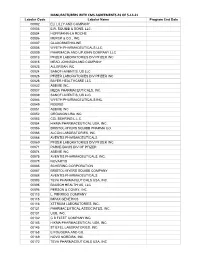
- Customer Rebate List Covering
- KEI Note Pharmaceutical Company R&D Cost Disclosures in SEC Filings
- 2020 Medicines in Development for Children
- Full Report PDF, 2.6 MB
- Rare Disease FEB14.Indd
- United States Securities and Exchange Commission Washington, D.C
- I, Virginie Hivert
- Spark Therapeutics CEO and Former Merck President to Keynote Veeva
- Disclosure Index
- Manufacturer's W/Start/End Dates and Sc Ind Appendix 1
- Document Nda 206488
- Fu N D C O M M En Ta
- Rebateable Manufacturers