Antibody Testing and Gene Therapy
Total Page:16
File Type:pdf, Size:1020Kb
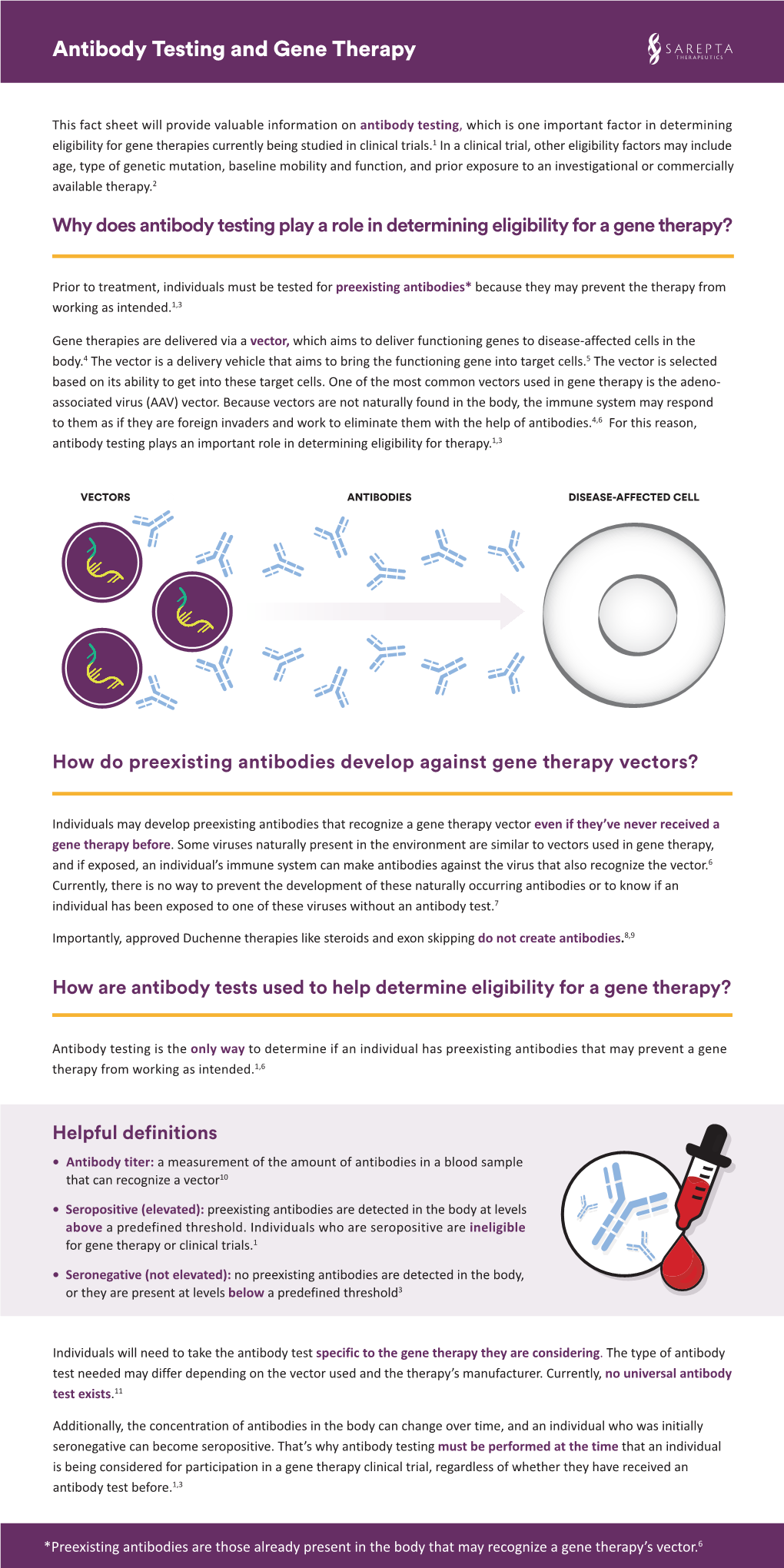
Load more
Recommended publications
-

NASDAQ Stock Market
Nasdaq Stock Market Friday, December 28, 2018 Name Symbol Close 1st Constitution Bancorp FCCY 19.75 1st Source SRCE 40.25 2U TWOU 48.31 21st Century Fox Cl A FOXA 47.97 21st Century Fox Cl B FOX 47.62 21Vianet Group ADR VNET 8.63 51job ADR JOBS 61.7 111 ADR YI 6.05 360 Finance ADR QFIN 15.74 1347 Property Insurance Holdings PIH 4.05 1-800-FLOWERS.COM Cl A FLWS 11.92 AAON AAON 34.85 Abiomed ABMD 318.17 Acacia Communications ACIA 37.69 Acacia Research - Acacia ACTG 3 Technologies Acadia Healthcare ACHC 25.56 ACADIA Pharmaceuticals ACAD 15.65 Acceleron Pharma XLRN 44.13 Access National ANCX 21.31 Accuray ARAY 3.45 AcelRx Pharmaceuticals ACRX 2.34 Aceto ACET 0.82 Achaogen AKAO 1.31 Achillion Pharmaceuticals ACHN 1.48 AC Immune ACIU 9.78 ACI Worldwide ACIW 27.25 Aclaris Therapeutics ACRS 7.31 ACM Research Cl A ACMR 10.47 Acorda Therapeutics ACOR 14.98 Activision Blizzard ATVI 46.8 Adamas Pharmaceuticals ADMS 8.45 Adaptimmune Therapeutics ADR ADAP 5.15 Addus HomeCare ADUS 67.27 ADDvantage Technologies Group AEY 1.43 Adobe ADBE 223.13 Adtran ADTN 10.82 Aduro Biotech ADRO 2.65 Advanced Emissions Solutions ADES 10.07 Advanced Energy Industries AEIS 42.71 Advanced Micro Devices AMD 17.82 Advaxis ADXS 0.19 Adverum Biotechnologies ADVM 3.2 Aegion AEGN 16.24 Aeglea BioTherapeutics AGLE 7.67 Aemetis AMTX 0.57 Aerie Pharmaceuticals AERI 35.52 AeroVironment AVAV 67.57 Aevi Genomic Medicine GNMX 0.67 Affimed AFMD 3.11 Agile Therapeutics AGRX 0.61 Agilysys AGYS 14.59 Agios Pharmaceuticals AGIO 45.3 AGNC Investment AGNC 17.73 AgroFresh Solutions AGFS 3.85 -

Guidelines with Regard to the Composition, Calculation and Management of the Index
INDEX METHODOLOGY Solactive Pharma Breakthrough Value Index Version 2.1 dated September 03, 2020 Contents Important Information 1. Index specifications 1.1 Short Name and ISIN 1.2 Initial Value 1.3 Distribution 1.4 Prices and Calculation Frequency 1.5 Weighting 1.6 Index Committee 1.7 Publication 1.8 Historical Data 1.9 Licensing 2. Composition of the Index 2.1 Selection of the Index Components 2.2 Ordinary Adjustment 2.3 Extraordinary Adjustment 3. Calculation of the Index 3.1 Index Formula 3.2 Accuracy 3.3 Adjustments 3.4 Dividends and other Distributions 3.5 Corporate Actions 3.6 Correction Policy 3.7 Market Disruption 3.8 Consequences of an Extraordinary Event 4. Definitions 5. Appendix 5.1 Contact Details 5.2 Calculation of the Index – Change in Calculation Method 2 Important Information This document (“Index Methodology Document”) contains the underlying principles and regulations regarding the structure and the operating of the Solactive Pharma Breakthrough Value Index. Solactive AG shall make every effort to implement regulations. Solactive AG does not offer any explicit or tacit guarantee or assurance, neither pertaining to the results from the use of the Index nor the Index value at any certain point in time nor in any other respect. The Index is merely calculated and published by Solactive AG and it strives to the best of its ability to ensure the correctness of the calculation. There is no obligation for Solactive AG – irrespective of possible obligations to issuers – to advise third parties, including investors and/or financial intermediaries, of any errors in the Index. -

SAREPTA THERAPEUTICS, INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-Q (Mark One) ☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended March 31, 2019 OR ☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number 001-14895 SAREPTA THERAPEUTICS, INC. (Exact name of registrant as specified in its charter) Delaware 93-0797222 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) 215 First Street, Suite 415 Cambridge, MA 02142 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (617) 274-4000 Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐ Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐ Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. -

05/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 04:25:50 Report Id 2794D052 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 05/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 04:25:50 REPORT ID 2794D052 PAGE: 01 ALPHA COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 07/01/2016 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 68782 (OSI) EYETECH 55513 AMGEN USA 00074 ABBOTT LABORATORIES 58406 AMGEN/IMMUNEX 68817 ABRAXIS BIOSCIENCE, LLC 53746 AMNEAL PHARMACEUTICALS 16729 ACCORD HEALTHCARE INCORPORATED 65162 AMNEAL PHARMACEUTICALS LLC 42192 ACELLA PHARMACEUTICALS, LLC 69238 AMNEAL PHARMACEUTICALS, LLC 10144 ACORDA THERAPEUTICS, INC. 53150 AMNEAL-AGILA, LLC 00472 ACTAVIS 00548 AMPHASTAR PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 69918 AMRING PHARMACEUTICALS, INC. 45963 ACTAVIS INC. 66780 AMYLIN PHARMACEUTICALS, INC. 46987 ACTAVIS KADIAN LLC 55724 ANACOR PHARMACEUTICALS 49687 ACTAVIS KADIAN LLC 10370 ANCHEN PHARMACEUTICALS, INC. 14550 ACTAVIS PHARMA MFGING PRIVATE LIMITED 43595 ANGELINI PHARMA, INC. 61874 ACTAVIS PHARMA, INC. 62559 ANIP ACQUISITION COMPANY 67767 ACTAVIS SOUTH ATLANTIC 54436 ANTARES PHARMA, INC. 66215 ACTELION PHARMACEUTICALS U.S., INC. 52609 APO-PHARMA USA, INC. 52244 ACTIENT PHARMACEUTICALS 60505 APOTEX CORP. 75989 ACTON PHARMACEUTICALS 63323 APP PHARMACEUTICALS, LLC. 69547 ADAPT PHARMA INC. 43485 APRECIA PHARMACEUTICALS COMPANY 76431 AEGERION PHARMACEUTICALS, INC. 42865 APTALIS PHARMA US, INC 50102 AFAXYS, INC. 58914 APTALIS PHARMA US, INC. 10572 AFFORDABLE PHARMACEUTICALS, LLC 13310 AR SCIENTIFIC, INC. 27241 AJANTA PHARMA LIMITED 08221 ARBOR PHARM IRELAND LIMITED 17478 AKORN INC 60631 ARBOR PHARMACEUTICALS IRELAND LIMITED 24090 AKRIMAX PHARMACEUTICALS LLC 24338 ARBOR PHARMACEUTICALS, INC. 68220 ALAVEN PHARMACEUTICAL, LLC 59923 AREVA PHARMACEUTICALS 00065 ALCON LABORATORIES, INC. 76189 ARIAD PHARMACEUTICALS, INC. 00998 ALCON LABORATORIES, INC. 24486 ARISTOS PHARMACEUTICALS, INC. -

Rebateable Manufacturers
Rebateable Labelers – July 2021 Manufacturers are responsible for updating their eligible drugs and pricing with CMS. Montana Healthcare Programs will not pay for an NDC not updated with CMS. Note: Some manufacturers on this list may have some NDCs that are covered and others that are not. Manufacturer ID Manufacturer Name 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC Page 1 of 19 Manufacturer ID Manufacturer Name 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. -

Cme Information for the Clinical Care Session Only
CME INFORMATION FOR THE CLINICAL CARE SESSION ONLY The University of Wisconsin–Madison Interprofessional Continuing Education Partnership (ICEP) and Cure SMA gratefully acknowledge the educational grants provided by Genentech and Novartis Gene Therapies. Target Audience Healthcare providers who diagnose and care for people with SMA, including physicians, nurse practitioners, physician assistants and nurses, pediatric and adult specialists including neurologists, pulmonologists, rehabilitation medicine, genetics, orthopedic surgery, palliative care, physical and occupational therapists, speech and language pathologists, pharmacists, nutritionists, genetic counselors, social workers, respiratory therapists, and trainees in the above disciplines. Educational Objectives After completing this activity, the participant should be better able to: 1. Demonstrate knowledge of multidisciplinary team roles and responsibilities in care management to optimize SMA outcomes. 2. Describe the existing and evolving treatment options for patients with SMA. 3. Describe the impact of new treatments on the SMA phenotype. 4. Analyze the treatment options for management of infants with SMA identified by SMA newborn screening. 5. Demonstrate knowledge of the risk for mental health complications of SMA. 6. Describe the application of mental health screening tools for SMA outpatient use. ACCREDITATION STATEMENT In support of improving patient care, this activity has been planned and implemented by the University of Wisconsin‒Madison Interprofessional Continuing Education Partnership (ICEP) and Cure SMA. The University of Wisconsin‒Madison ICEP is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC) to provide continuing education for the healthcare team. CREDIT DESIGNATION STATEMENTS American Medical Association (AMA) The University of Wisconsin–Madison ICEP designates this live activity for a maximum of 5.5 AMA PRA Category 1 Credits™. -

The Bottom 99
The Top 100 January, 2015 A list of stocks topping our custom 'torpedo’ screen. Updated monthly. AWAY Homeaway, Inc. Consumer Discretionary DWA DreamWorks Animation SKG, Inc. Class A Consumer Discretionary DXLG Destination XL Group, Inc. Consumer Discretionary NXST Nexstar Broadcasting Group, Inc. Class A Consumer Discretionary TSLA Tesla Motors, Inc. Consumer Discretionary ZQK Quiksilver, Inc. Consumer Discretionary CMLP Crestwood Midstream Partners LP Energy LNG Cheniere Energy, Inc. Energy SZYM Solazyme, Inc. Energy ACAD ACADIA Pharmaceuticals Inc. Health Care AKRX Akorn, Inc. Health Care ALIM Alimera Sciences, Inc. Health Care ALNY Alnylam Pharmaceuticals, Inc Health Care ARWR Arrowhead Research Corporation Health Care ATHN athenahealth, Inc. Health Care ATHX Athersys, Inc. Health Care AUXL Auxilium Pharmaceuticals, Inc. Health Care BDSI BioDelivery Sciences International, Inc. Health Care CERS Cerus Corporation Health Care CLDX Celldex Therapeutics, Inc. Health Care CPHD Cepheid Health Care CSII Cardiovascular Systems, Inc. Health Care CSU Capital Senior Living Corporation Health Care DRRX DURECT Corporation Health Care DYAX Dyax Corp. Health Care EBIO Eleven Biotherapeutics, Inc. Health Care EGLT Egalet Corporation Health Care EPZM Epizyme, Inc. Health Care EXAS Exact Sciences Corporation Health Care FCSC Fibrocell Science, Inc. Health Care FLDM Fluidigm Corporation Health Care GEVA Synageva BioPharma Corp. Health Care HALO Halozyme Therapeutics, Inc. Health Care ICPT Intercept Pharmaceuticals, Inc. Health Care IDRA Idera Pharmaceuticals, Inc. Health Care INSM Insmed Incorporated Health Care IRWD Ironwood Pharmaceuticals, Inc. Class A Health Care ISR IsoRay, Inc. Health Care KERX Keryx Biopharmaceuticals, Inc. Health Care KPTI Karyopharm Therapeutics, Inc. Health Care KYTH KYTHERA Biopharmaceuticals, Inc. Health Care MDCO Medicines Company Health Care MDSO Medidata Solutions, Inc. -

Rare Diseases Whitepaper
Future Treatment Approaches for Rare Congenital and Genetic Diseases WHITEPAPER Author: Anna Osborne Principal Consultant, Informa Pharma Custom Intelligence Introduction Many biotech and pharmaceuticals companies more, regulatory and legislative initiatives such as have prioritized drug development for rare Breakthrough Therapy designation, which makes congenital and genetic diseases over the past it easier to work with the FDA on tailored trial few years given the high unmet need, rapidly designs, and the Orphan Drug Act, which provides advancing science, and favorable clinical seven years of regulatory exclusivity, have development paths. Rare congenital and encouraged development in this space. genetic diseases often have severe or even fatal manifestations, with few treatments available, In this whitepaper, we review the current market but emerging genetic data and new treatment landscape for rare congenital and genetic diseases modalities, such as gene therapy, are rendering and look forward to what treatment approaches monogenic diseases more tractable. What’s might be available soon. 2 / August 2020 © Informa UK Ltd 2020 (Unauthorized photocopying prohibited.) Approved therapies for rare congenital and genetic diseases The focus of this analysis is limited to diseases by GARD, and emphasizes the huge unmet need that are classified as rare congenital and genetic that exists. Diseases with the largest number of diseases by the National Center for Advancing approved therapies include dwarfism, Lennox- Translational Sciences’ (part of the NIH) Genetic Gastaut syndrome, and cystic fibrosis (Figure 1). and Rare Diseases Information Center (GARD)1. However, even for these diseases the number According to Informa’s Pharmaprojects, only of approved therapies is very small compared 74 rare congenital or genetic diseases have an to more prevalent conditions, and the need for approved therapy. -

MDA2020 Mitelman Sarepta Study 405 FINAL.Pdf
Combined Prospective and Retrospective Analysis of Duchenne Muscular Dystrophy Patient Outcomes Following 7 Years of Eteplirsen Treatment Compared With Natural History External Control Cohorts Olga Mitelman,1 Hoda Z. Abdel‐Hamid,2 Barry J. Byrne,3 Anne M. Connolly,4 Peter Heydemann,5 Crystal Proud,6 Perry B. Shieh,7 Kathryn R. Wagner,8 Ashish Dugar,1 Sourav Santra,1 James Signorovitch,9 Katherine Tsai,1 Jerry R. Mendell4 1Sarepta Therapeutics, Inc., Cambridge, MA, USA; 2UPMC Children’s Hospital of Pittsburgh, Pittsburgh, PA, USA; 3University of Florida, Gainesville, FL, USA; 4Nationwide Children’s Hospital, The Ohio State University College of Medicine, Columbus, OH, USA; 5Rush University Medical Center, Chicago, IL, USA; 6Children’s Hospital of The King’s Daughters, Norfolk, VA, USA; 7David Geffen School of Medicine at UCLA, Los Angeles, CA, USA; 8Kennedy Krieger Institute, Johns Hopkins School of Medicine, Baltimore, MD, USA; 9Analysis Group, Inc., Boston, MA, USA BACKGROUND RESULTS Figure 3. Descriptive Analysis of Age at LOA in Eteplirsen‐Treated Patients and Published Natural History Cohort Data12,13 • Duchenne muscular dystrophy (DMD) is a fatal, X‐linked Patients 15.2 neuromuscular disease caused by mutations in the dystrophin gene • Of 12 patients enrolled in Study 201/202, 10 underwent chart 15 13.0 (DMD)1,2 review in Study 405 12.0 (years) • Eteplirsen binds to exon 51 of dystrophin pre‐mRNA to allow • Study 405 participants had comparable demographics and disease a 10 LOA skipping of exon 51, restoring the mRNA reading frame -
MANUFACTURERS with CMS AGREEMENTS AS of 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY and COMPANY 00003 E.R
MANUFACTURERS WITH CMS AGREEMENTS AS OF 5-14-21 Labeler Code Labeler Name Program End Date 00002 ELI LILLY AND COMPANY 00003 E.R. SQUIBB & SONS, LLC. 00004 HOFFMANN-LA ROCHE 00006 MERCK & CO., INC. 00007 GLAXOSMITHKLINE 00008 WYETH PHARMACEUTICALS LLC, 00009 PHARMACIA AND UPJOHN COMPANY LLC 00013 PFIZER LABORATORIES DIV PFIZER INC 00015 MEAD JOHNSON AND COMPANY 00023 ALLERGAN INC 00024 SANOFI-AVENTIS, US LLC 00025 PFIZER LABORATORIES DIV PFIZER INC 00026 BAYER HEALTHCARE LLC 00032 ABBVIE INC. 00037 MEDA PHARMACEUTICALS, INC. 00039 SANOFI-AVENTIS, US LLC 00046 WYETH PHARMACEUTICALS INC. 00049 ROERIG 00051 ABBVIE INC 00052 ORGANON USA INC. 00053 CSL BEHRING L.L.C. 00054 HIKMA PHARMACEUTICAL USA, INC. 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00065 ALCON LABORATORIES, INC. 00068 AVENTIS PHARMACEUTICALS 00069 PFIZER LABORATORIES DIV PFIZER INC 00071 PARKE-DAVIS DIV OF PFIZER 00074 ABBVIE INC 00075 AVENTIS PHARMACEUTICALS, INC. 00078 NOVARTIS 00085 SCHERING CORPORATION 00087 BRISTOL-MYERS SQUIBB COMPANY 00088 AVENTIS PHARMACEUTICALS 00093 TEVA PHARMACEUTICALS USA, INC. 00095 BAUSCH HEALTH US, LLC 00096 PERSON & COVEY, INC. 00113 L. PERRIGO COMPANY 00115 IMPAX GENERICS 00116 XTTRIUM LABORATORIES, INC. 00121 PHARMACEUTICAL ASSOCIATES, INC. 00131 UCB, INC. 00132 C B FLEET COMPANY INC 00143 HIKMA PHARMACEUTICAL USA, INC. 00145 STIEFEL LABORATORIES, INC, 00168 E FOUGERA AND CO. 00169 NOVO NORDISK, INC. 00172 TEVA PHARMACEUTICALS USA, INC Labeler Code Labeler Name Program End Date 00173 GLAXOSMITHKLINE 00178 MISSION PHARMACAL COMPANY 00185 EON LABS, INC. 00186 ASTRAZENECA PHARMACEUTICALS LP 00187 BAUSCH HEALTH US, LLC. 00206 WYETH PHARMACEUTICALS LLC, 00224 KONSYL PHARMACEUTICALS, INC. 00225 B. F. ASCHER AND COMPANY, INC. 00228 ACTAVIS PHARMA, INC. -

Sarepta, FDA and the Dangers of Strong Early Results
REGULATORY UPDATE BUSINESS & FINANCE MANUFACTURING EMA, FDA Get Together On Drugs Lundbeck Takes A Singular View FDA Making A Generic Case For Eligible For PRIME And Breakthrough Toward Tackling Challenging CNS Continuous Manufacturing, p. 23 Designation, p. 19 Disorders, p. 5 Pharma intelligence Pinkwww.ThePinkSheet.com SheetVol. 78 / No. 18 May 2, 2016 informa tinue to work with FDA as it completes the Sarepta, FDA And The Dangers eteplirsen review and that it remains com- mitted to gaining approval for a Duchenne treatment. Of Strong Early Results Debra Miller, CEO and founder of the advocacy group CureDuchenne, said in an DERRICK GINGERY [email protected] interview with “The Pink Sheet” that she wants to make sure Duchenne patients and arepta Therapeutics Inc.’s proposed advocates remain focused on dealing with Duchenne muscular dystrophy treat- the agency’s concerns. Sment eteplirsen may have been “We just work as hard as we can to help doomed by the optimism created from its Sarepta and make sure this doesn’t happen early study results, which it turned out could again and design trials that FDA will like,” not be replicated. she said. And now the company finds itself in a pre- The committee’s decision was made diffi- carious situation as it looks for a way to gen- cult by the emotional and admittedly com- erate additional scientific data that match pelling testimony from dozens of patients, the overwhelmingly positive anecdotal evi- parents and advocates (“Patients Can’t Res- dence of efficacy. cue Sarepta’s Eteplirsen” — “The Pink Sheet” FDA made no secret of the fact that it had DAILY, April 25, 2016). -

Sarepta Therapeutics, Inc. Annual Report 2016
Sarepta Therapeutics, Inc. Annual Report 2016 Form 10-K (NASDAQ:SRPT) Published: April 29th, 2016 PDF generated by stocklight.com UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K/A (Amendment No. 1) (Mark One) x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2015 Or o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission file number: 001-14895 Sarepta Therapeutics, Inc. (Exact name of registrant as specified in its charter) Delaware 93-0797222 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification Number) 215 First Street Suite 415 Cambridge, MA 02142 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (617) 274-4000 Securities registered pursuant to Section 12(b) of the Act: Tile of Each Class Name of Exchange on Which Registered Common Stock, $0.0001 par value The NASDAQ Stock Market LLC (The NASDAQ Global Select Market) Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.