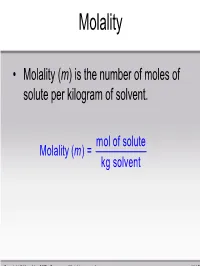
Molality
Top View
- Solutions and Colloids 603
- Solubility Science: Principles and Practice Prof Steven Abbott
- From Molarity to Molality and Vice Versa
- Gas Concentrations, Ph and Molality
- Properties of Solutions
- Studies of Osmotic Coefficients and Volumetric Behaviour on Aqueous Solutions of ~-Cyclodextrin at 298.15 K
- Sample Quiz and Test Questions – Chapter 4
- IMPORTANT CHEMICAL CONCEPTS: SOLUTIONS, CONCENTRATIONS, STOICHIOMETRY I. Introduction A. Course Outline Will Be Reviewed. B
- Chapter 13 Properties of Solutions Classification of Matter
- Consideration of Long and Middle Range Interaction on the Calculation of Activities for Binary Polymer Solutions
- Appendix N CONCENTRATION UNITS
- Solutions and Their Properties
- MOLALITY (M) and MOLE FRACTION (Xi)
- Thennodynanllcsandthe Other Chemical Engineering Sciences: Old Models for New Chemical Processes
- Solutions and Units of Concentration
- Solutions 14.Notebook 1 January 26, 2016
- Molar Mass by Freezing Point Depression OBJECTIVES
- Chapter 14 Solutions and Their Behavior