Molarity Versus Molality Concentration of Solutions SCIENTIFIC
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Solutes and Solution
Solutes and Solution The first rule of solubility is “likes dissolve likes” Polar or ionic substances are soluble in polar solvents Non-polar substances are soluble in non- polar solvents Solutes and Solution There must be a reason why a substance is soluble in a solvent: either the solution process lowers the overall enthalpy of the system (Hrxn < 0) Or the solution process increases the overall entropy of the system (Srxn > 0) Entropy is a measure of the amount of disorder in a system—entropy must increase for any spontaneous change 1 Solutes and Solution The forces that drive the dissolution of a solute usually involve both enthalpy and entropy terms Hsoln < 0 for most species The creation of a solution takes a more ordered system (solid phase or pure liquid phase) and makes more disordered system (solute molecules are more randomly distributed throughout the solution) Saturation and Equilibrium If we have enough solute available, a solution can become saturated—the point when no more solute may be accepted into the solvent Saturation indicates an equilibrium between the pure solute and solvent and the solution solute + solvent solution KC 2 Saturation and Equilibrium solute + solvent solution KC The magnitude of KC indicates how soluble a solute is in that particular solvent If KC is large, the solute is very soluble If KC is small, the solute is only slightly soluble Saturation and Equilibrium Examples: + - NaCl(s) + H2O(l) Na (aq) + Cl (aq) KC = 37.3 A saturated solution of NaCl has a [Na+] = 6.11 M and [Cl-] = -

Parametrisation in Electrostatic DPD Dynamics and Applications
Parametrisation in electrostatic DPD Dynamics and Applications E. Mayoraly and E. Nahmad-Acharz February 19, 2016 y Instituto Nacional de Investigaciones Nucleares, Carretera M´exico-Toluca S/N, La Marquesa Ocoyoacac, Edo. de M´exicoC.P. 52750, M´exico z Instituto de Ciencias Nucleares, Universidad Nacional Aut´onomade M´exico, Apartado Postal 70-543, 04510 M´exico DF, Mexico abstract A brief overview of mesoscopic modelling via dissipative particle dynamics is presented, with emphasis on the appropriate parametrisation and how to cal- culate the relevant parameters for given realistic systems. The dependence on concentration and temperature of the interaction parameters is also considered, as well as some applications. 1 Introduction In a colloidal dispersion, the stability is governed by the balance between Van der Waals attractive forces and electrostatic repulsive forces, together with steric mechanisms. Being able to model their interplay is of utmost importance to predict the conditions for colloidal stability, which in turn is of major interest in basic research and for industrial applications. Complex fluids are composed typically at least of one or more solvents, poly- meric or non-polymeric surfactants, and crystalline substrates onto which these surfactants adsorb. Neutral polymer adsorption has been extensively studied us- ing mean-field approximations and assuming an adsorbed polymer configuration of loops and tails [1,2,3,4]. Different mechanisms of adsorption affecting the arXiv:1602.05935v1 [physics.chem-ph] 18 Feb 2016 global -

THE SOLUBILITY of GASES in LIQUIDS Introductory Information C
THE SOLUBILITY OF GASES IN LIQUIDS Introductory Information C. L. Young, R. Battino, and H. L. Clever INTRODUCTION The Solubility Data Project aims to make a comprehensive search of the literature for data on the solubility of gases, liquids and solids in liquids. Data of suitable accuracy are compiled into data sheets set out in a uniform format. The data for each system are evaluated and where data of sufficient accuracy are available values are recommended and in some cases a smoothing equation is given to represent the variation of solubility with pressure and/or temperature. A text giving an evaluation and recommended values and the compiled data sheets are published on consecutive pages. The following paper by E. Wilhelm gives a rigorous thermodynamic treatment on the solubility of gases in liquids. DEFINITION OF GAS SOLUBILITY The distinction between vapor-liquid equilibria and the solubility of gases in liquids is arbitrary. It is generally accepted that the equilibrium set up at 300K between a typical gas such as argon and a liquid such as water is gas-liquid solubility whereas the equilibrium set up between hexane and cyclohexane at 350K is an example of vapor-liquid equilibrium. However, the distinction between gas-liquid solubility and vapor-liquid equilibrium is often not so clear. The equilibria set up between methane and propane above the critical temperature of methane and below the criti cal temperature of propane may be classed as vapor-liquid equilibrium or as gas-liquid solubility depending on the particular range of pressure considered and the particular worker concerned. -

Introduction to the Solubility of Liquids in Liquids
INTRODUCTION TO THE SOLUBILITY OF LIQUIDS IN LIQUIDS The Solubility Data Series is made up of volumes of comprehensive and critically evaluated solubility data on chemical systems in clearly defined areas. Data of suitable precision are presented on data sheets in a uniform format, preceded for each system by a critical evaluation if more than one set of data is available. In those systems where data from different sources agree sufficiently, recommended values are pro posed. In other cases, values may be described as "tentative", "doubtful" or "rejected". This volume is primarily concerned with liquid-liquid systems, but related gas-liquid and solid-liquid systems are included when it is logical and convenient to do so. Solubilities at elevated and low 'temperatures and at elevated pressures may be included, as it is considered inappropriate to establish artificial limits on the data presented. For some systems the two components are miscible in all proportions at certain temperatures or pressures, and data on miscibility gap regions and upper and lower critical solution temperatures are included where appropriate and if available. TERMINOLOGY In this volume a mixture (1,2) or a solution (1,2) refers to a single liquid phase containing components 1 and 2, with no distinction being made between solvent and solute. The solubility of a substance 1 is the relative proportion of 1 in a mixture which is saturated with respect to component 1 at a specified temperature and pressure. (The term "saturated" implies the existence of equilibrium with respect to the processes of mass transfer between phases) • QUANTITIES USED AS MEASURES OF SOLUBILITY Mole fraction of component 1, Xl or x(l): ml/Ml nl/~ni = r(m.IM.) '/. -

Hydrophobic Forces Between Protein Molecules in Aqueous Solutions of Concentrated Electrolyte
LBNL-47348 Pre print !ERNEST ORLANDO LAWRENCE BERKELEY NATIONAL LABORATORY Hydrophobic Forces Between Protein Molecules in Aqueous Solutions of Concentrated Electrolyte R.A. Curtis, C. Steinbrecher, M. Heinemann, H.W. Blanch, and J .M. Prausnitz Chemical Sciences Division January 2001 Submitted to Biophysical]ournal - r \ ' ,,- ' -· ' .. ' ~. ' . .. "... i ' - (_ '·~ -,.__,_ .J :.r? r Clz r I ~ --.1 w ~ CD DISCLAIMER This document was prepared as an account of work sponsored by the United States Government. While this document is believed to contain correct information, neither the United States Government nor any agency thereof, nor the Regents of the University of California, nor any of their employees, makes any warranty, express or implied, or assumes any legal responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by its trade name, trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof, or the Regents of the University of California. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof or the Regents of the University of California. LBNL-47348 Hydrophobic Forces Between Protein Molecules in Aqueous Solutions of Concentrated Electrolyte R. A. Curtis, C. Steinbrecher. M. Heinemann, H. W. Blanch and J. M. Prausnitz Department of Chemical Engineering University of California and Chemical Sciences Division Lawrence Berkeley National Laboratory University of California Berkeley, CA 94720, U.S.A. -

Δtb = M × Kb, Δtf = M × Kf
8.1HW Colligative Properties.doc Colligative Properties of Solvents Use the Equations given in your notes to solve the Colligative Property Questions. ΔTb = m × Kb, ΔTf = m × Kf Freezing Boiling K K Solvent Formula Point f b Point (°C) (°C/m) (°C/m) (°C) Water H2O 0.000 100.000 1.858 0.521 Acetic acid HC2H3O2 16.60 118.5 3.59 3.08 Benzene C6H6 5.455 80.2 5.065 2.61 Camphor C10H16O 179.5 ... 40 ... Carbon disulfide CS2 ... 46.3 ... 2.40 Cyclohexane C6H12 6.55 80.74 20.0 2.79 Ethanol C2H5OH ... 78.3 ... 1.07 1. Which solvent’s freezing point is depressed the most by the addition of a solute? This is determined by the Freezing Point Depression constant, Kf. The substance with the highest value for Kf will be affected the most. This would be Camphor with a constant of 40. 2. Which solvent’s freezing point is depressed the least by the addition of a solute? By the same logic as above, the substance with the lowest value for Kf will be affected the least. This is water. Certainly the case could be made that Carbon disulfide and Ethanol are affected the least as they do not have a constant. 3. Which solvent’s boiling point is elevated the least by the addition of a solute? Water 4. Which solvent’s boiling point is elevated the most by the addition of a solute? Acetic Acid 5. How does Kf relate to Kb? Kf > Kb (fill in the blank) The freezing point constant is always greater. -

Topic720 Composition: Mole Fraction: Molality: Concentration a Solution Comprises at Least Two Different Chemical Substances
Topic720 Composition: Mole Fraction: Molality: Concentration A solution comprises at least two different chemical substances where at least one substance is in vast molar excess. The term ‘solution’ is used to describe both solids and liquids. Nevertheless the term ‘solution’ in the absence of the word ‘solid’ refers to a liquid. Chemists are particularly expert at identifying the number and chemical formulae of chemical substances present in a given closed system. Here we explore how the chemical composition of a given system is expressed. We consider a simple system prepared using water()l and urea(s) at ambient temperature and pressure. We designate water as chemical substance 1 and urea as chemical substance j, so that the closed system contains an aqueous solution. The amounts of the two substances are given by n1 = = ()wM11 and nj ()wMjj where w1 and wj are masses; M1 and Mj are the molar masses of the two chemical substances. In these terms, n1 and nj are extensive variables. = ⋅ + ⋅ Mass of solution, w n1 M1 n j M j (a) = ⋅ Mass of solvent, w1 n1 M1 (b) = -1 For water, M1 0.018 kg mol . However in reviewing the properties of solutions, chemists prefer intensive composition variables. Mole Fraction The mole fractions of the two substances x1 and xj are given by the following two equations: =+ =+ xnnn111()j xnnnjj()1 j (c) += Here x1 x j 10. In general terms for a system comprising i - chemical substances, the mole fraction of substance k is given by equation (d). ji= = xnkk/ ∑ n j (d) j=1 ji= = Hence ∑ x j 10. -
Mol of Solute Molality ( ) = Kg Solvent M
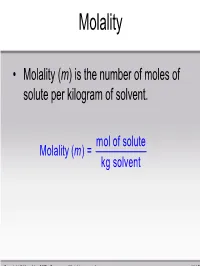
Molality • Molality (m) is the number of moles of solute per kilogram of solvent. mol of solute Molality (m ) = kg solvent Copyright © Houghton Mifflin Company. All rights reserved. 11 | 51 Sample Problem Calculate the molality of a solution of 13.5g of KF dissolved in 250. g of water. mol of solute m = kg solvent 1 m ol KF ()13.5g 58.1 = 0.250 kg = 0.929 m Copyright © Houghton Mifflin Company. All rights reserved. 11 | 52 Mole Fraction • Mole fraction (χ) is the ratio of the number of moles of a substance over the total number of moles of substances in solution. number of moles of i χ = i total number of moles ni = nT Copyright © Houghton Mifflin Company. All rights reserved. 11 | 53 Sample Problem -Conversions between units- • ex) What is the molality of a 0.200 M aluminum nitrate solution (d = 1.012g/mL)? – Work with 1 liter of solution. mass = 1012 g – mass Al(NO3)3 = 0.200 mol × 213.01 g/mol = 42.6 g ; – mass water = 1012 g -43 g = 969 g 0.200mol Molality==0.206 mol / kg 0.969kg Copyright © Houghton Mifflin Company. All rights reserved. 11 | 54 Sample Problem Calculate the mole fraction of 10.0g of NaCl dissolved in 100. g of water. mol of NaCl χ=NaCl mol of NaCl + mol H2 O 1 mol NaCl ()10.0g 58.5g NaCl = 1 m ol NaCl 1 m ol H2 O ()10.0g+ () 100.g H2 O 58.5g NaCl 18.0g H2 O = 0.0299 Copyright © Houghton Mifflin Company. -

Study of Interfacial Tension Between an Organic Solvent and Aqueous Electrolyte Solutions Using Electrostatic Dissipative Particle Dynamics Simulations
1 Study of Interfacial Tension between an Organic Solvent and Aqueous Electrolyte Solutions Using Electrostatic Dissipative Particle Dynamics Simulations. E. Mayoral (1), E. Nahmad-Achar(2) 1 Instituto Nacional de Investigaciones Nucleares, Carretera México-Toluca s/n, La Marquesa Ocoyoacac, Estado de México CP 52750, México. Email address: [email protected] 2 Instituto de Ciencias Nucleares, Universidad Nacional Autónoma de México (UNAM), Apartado Postal 70-543, 04510 México D.F. Email address: [email protected] ABSTRACT The study of the modification of interfacial properties between an organic solvent and aqueous electrolyte solutions is presented by using electrostatic Dissipative Particle Dynamics (DPD) simulations. In this article the parametrization for the DPD repulsive parameters aij for the electrolyte components is calculated considering the dependence of the Flory-Huggins χ parameter on the concentration and the kind of electrolyte added, by means of the activity coefficients. In turn, experimental data was used to obtain the activity coefficients of the electrolytes as a function of their concentration in order to estimate the χ parameters and then the aij coefficients. We validate this parametrization through the study of the interfacial tension in a mixture of n-dodecane and water, varying the concentration of different inorganic salts (NaCl, KBr, Na2SO4 and UO2Cl2). The case of HCl in the mixture n-dodecane/water was also analyzed and the results presented. Our simulations reproduce the experimental data in good agreement with previous work, showing that the use of activity coefficients to obtain the repulsive DPD parameters aij as a function of concentration is a good alternative for these kinds of systems. -

Chapter 11 – Colloids – Larger Particles but Still Uniform (Milk, Fog)
Solutions • Homogeneous mixtures: – Solutions – ions or molecules (small particles) Chapter 11 – Colloids – larger particles but still uniform (milk, fog) Solutions and Their Properties 1 The Solution Process The Solution Process • During dissolution, some forces are • Solvent-solvent interactions broken and new forces are created • Solute-solute interactions • Solvent-solute interactions ∆∆∆ ∆∆∆ ∆∆∆ ∆∆∆ Hsoln = H1 + H2 + H3 Figure 12.2 3 4 The Solution Process Hot and Cold Packs • Generally, • Hot packs – dissolving solid releases • if the solute is more strongly attracted heat ∆∆∆ to the solvent attraction (than to itself – – CaCl 2 (s) Hsoln = -81.3 kJ/mol ∆∆∆ weak IMF), then dissolution is – MgSO 4 (s) Hsoln = -91.2 kJ/mol ∆∆∆ favorable; exothermic ( Hsoln < 0) • Cold packs – dissolving solid absorbs • if the solute is more strongly attracted heat ∆∆∆ to itself (than to the solvent – strong – NH 4NO 3 (s) Hsoln = +25.7 kJ/mol IMF), then dissolution is not favorable; ∆∆∆ endothermic ( Hsoln > 0) Ways of Expressing Concentration Concentration Units • Variety of units • Molarity – Most commonly used is M (molarity) – Molarity = moles solute / liter solution = mol/L – Also ppm, mole fraction, molality, and Normality – Depends on temperature; density of liquids o changes with temperature (d H2O at 20 C = 3 • Qualitative terms relating to solubility 0.9982 g/cm ) – insoluble, slightly soluble, soluble, very soluble – Molarity: – <0.1 g/100g >2 g/100 g – Ex: 5.0 g NaCl in water that gives a volume of 251mL • Other comparative terms: – Ans: -

Lecture 26 Solutions I Tutorial 1) How Many Grams of Mgso4·9H2O Are Needed to Prepare 125 Ml of 0.200 M Magnesium Sulfate?
www.apchemsolutions.com Lecture 26 Solutions I Tutorial 1) How many grams of MgSO4·9H2O are needed to prepare 125 mL of 0.200 M magnesium sulfate? 1L 0.200 mol MgSO42⋅ 9H O 282.56 g MgSO42⋅ 9H O 125 mL× × × =7.06 g MgSO42⋅ 9H O 1000 mL 1L 1mol MgSO42⋅ 9H O 2) 251 mL of 0.45 M HCl is added to 455 mL of distilled water. What is the molarity of the final solution? (Hint: find moles of HCl and total volume of the final solution) 1L 0.45mol HCl 251mL Solution× × =0.11mol HCl 1000mL 1L Solution 251 mL + 455 mL = 706 mL 0.11mol HCl Molarity = =0.16 M HCl 0.706L 3) A 5.75 g sample of KOH is dissolved in 425 mL of water. Find the molality of the solution. 1mol KOH 0.102 mol KOH 5.75g KOH × = 0.102 mol KOH molality = =0.240 m 56.11g KOH 0.425 kg water 4) Find the molality of a 5.25 M LiBr solution that has a density of 1.25 g/mL. 5.25 mol LiBr 86.84 g LiBr 1 L solution× × = 456 g LiBr 1 L solution 1mol LiBr 1000 mL 1.25 g solution 1L solution× × = 1250 g solution 1L 1 mL solution mass solvent = mass solution - mass solute mass solvent = 1250g solution - 456g LiBr = 794 g solvent moles solute 5.25 mol LiBr molality = = = 6.61 m kg solvent 0.794 kg solvent © 2009, 2008 AP Chem Solutions. All rights reserved. 1 www.apchemsolutions.com 5) Find the mole fraction of glucose, C6H12O6, in a 2.1 m solution of glucose and water. -

Molality = Moles of Solute/Kg of Solvent (M) Moles of Solute/Kg of Solvent (M)
5/2/2010 Properties of Solutions Copyright © Houghton Mifflin Company. All rights reserved. 17a–2 Figure 17.2: (a) Enthalpy of solution Hsoln has a negative sign (the process is Figure 17.1: The formation of a liquid exothermic) if Step 3 releases more energy than is required by Steps 1 and 2. (b) Hsoln has a positive sign (the process is endothermic) if Stpes 1 and 2 require solution can be divided into three steps more energy than is released in Step 3. Copyright © Houghton Mifflin Company. All rights reserved. 17a–3 Copyright © Houghton Mifflin Company. All rights reserved. 17a–4 Concentration Molarity = Moles of solute/Liters of Solution (M) Entropy Usually Favors Making Solutions Molality = Because There Are More Moles of solute/Kg of Solvent (m) Possible Arrangements of the Atoms or Mole Fraction= Molecules Moles solute/total number of moles Mass %= Mass solute/total mass x 100 Copyright © Houghton Mifflin Company. All rights reserved. 17a–5 Copyright © Houghton Mifflin Company. All rights reserved. 17a–6 1 5/2/2010 Concentration Concentration Molarity = Molarity = Moles of solute/Liters of Solution (M) Moles of solute/Liters of Solution (M) Molality = Molality = Moles of solute/Kg of Solvent (m) Moles of solute/Kg of Solvent (m) Mole Fraction= Mole Fraction= Moles solute/total number of moles Moles solute/total number of moles Mass %= Mass %= Mass solute/total mass x 100 Mass solute/total mass x 100 Copyright © Houghton Mifflin Company. All rights reserved. 17a–7 Copyright © Houghton Mifflin Company. All rights reserved. 17a–8 A sample of NaNO3 weighing 8.5 grams is placed in a 500 ml volumetric flask and distilled water was added to the mark on the Molarity = neck of the flask.