Petrosal Morphology and Cochlear Function in Mesozoic Stem
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution of the Patellar Sesamoid Bone in Mammals
A peer-reviewed version of this preprint was published in PeerJ on 21 March 2017. View the peer-reviewed version (peerj.com/articles/3103), which is the preferred citable publication unless you specifically need to cite this preprint. Samuels ME, Regnault S, Hutchinson JR. 2017. Evolution of the patellar sesamoid bone in mammals. PeerJ 5:e3103 https://doi.org/10.7717/peerj.3103 Evolution of the patellar sesamoid bone in mammals Mark E Samuels 1, 2 , Sophie Regnault 3 , John R Hutchinson Corresp. 3 1 Department of Medicine, University of Montreal, Montreal, Quebec, Canada 2 Centre de Recherche du CHU Ste-Justine, Montreal, Quebec, Canada 3 Structure & Motion Laboratory, Department of Comparative Biomedical Sciences, The Royal Veterinary College, Hatfield, Hertfordshire, United Kingdom Corresponding Author: John R Hutchinson Email address: [email protected] The patella is a sesamoid bone located in the major extensor tendon of the knee joint, in the hindlimb of many tetrapods. Although numerous aspects of knee morphology are ancient and conserved among most tetrapods, the evolutionary occurrence of an ossified patella is highly variable. Among extant (crown clade) groups it is found in most birds, most lizards, the monotreme mammals and almost all placental mammals, but it is absent in most marsupial mammals as well as many reptiles. Here we integrate data from the literature and first-hand studies of fossil and recent skeletal remains to reconstruct the evolution of the mammalian patella. We infer that bony patellae most likely evolved between four to six times in crown group Mammalia: in monotremes, in the extinct multituberculates, in one or more stem-mammal genera outside of therian or eutherian mammals, and up to three times in therian mammals. -

A New Gobiconodontid Mammal from the Early Cretaceous of Spain and Its Palaeogeographic Implications
A new gobiconodontid mammal from the Early Cretaceous of Spain and its palaeogeographic implications Gloria Cuenca-Bescós and José I. Canudo Acta Palaeontologica Polonica 48 (4), 2003: 575-582 A new gobiconodontid from Vallipón (Teruel, Spain) represents the first record of this family in Europe. The site has a diverse fossil assemblage mainly composed of isolated bones and teeth probably accumulated by tidal action and water streams in an ancient beach of upper Barremian, in the transitional marine-continental sediments of the Artoles Formation. The new gobiconodontid consist of an isolated upper molar, smaller in size than that element in other gobiconodontids, with a robust cusp A, characterised by lateral bulges on each mesial and distal flanges of that cusp, and a discontinuous cingulum raised at the lingual side. The oclusal outline is smooth compared with Gobiconodon borissiaki, Gobiconodon hoburensis, or Gobiconodon ostromi. The Gobiconodontidae record is exclusively Laurasiatic. The oldest gobiconodontid fossil remains are Hauterivian; though their probable origin has to be found at the Late Jurassic in Central Asia (as inferred from derived character of the first gobiconodontids as well as phylogenetic relationships). At the end of the Early Cretaceous they expanded throughout Laurasia as indicated by findings in Asia, North America and Spain. Two dispersion events spread gobiconodontids: to the West (Europe) in the Barremian and to the East (North America) during the Aptian/Albian. Key words: Cretaceous, Barremian, Mammalia, Gobiconodontidae, Europe, palaeogeography. Gloria Cuenca−Bescós [[email protected]], José Ignacio Canudo [[email protected]], Area y Museo de Paleontología, Departamento de Ciencias de la Tierra, Universidad de Zaragoza, 50009 Zaragoza, Spain. -

Miocene Mammal Reveals a Mesozoic Ghost Lineage on Insular New Zealand, Southwest Pacific
Miocene mammal reveals a Mesozoic ghost lineage on insular New Zealand, southwest Pacific Trevor H. Worthy*†, Alan J. D. Tennyson‡, Michael Archer§, Anne M. Musser¶, Suzanne J. Hand§, Craig Jonesʈ, Barry J. Douglas**, James A. McNamara††, and Robin M. D. Beck§ *School of Earth and Environmental Sciences, Darling Building DP 418, Adelaide University, North Terrace, Adelaide 5005, South Australia, Australia; ‡Museum of New Zealand Te Papa Tongarewa, P.O. Box 467, Wellington 6015, New Zealand; §School of Biological, Earth and Environmental Sciences, University of New South Wales, New South Wales 2052, Australia; ¶Australian Museum, 6-8 College Street, Sydney, New South Wales 2010, Australia; ʈInstitute of Geological and Nuclear Sciences, P.O. Box 30368, Lower Hutt 5040, New Zealand; **Douglas Geological Consultants, 14 Jubilee Street, Dunedin 9011, New Zealand; and ††South Australian Museum, Adelaide, South Australia 5000, Australia Edited by James P. Kennett, University of California, Santa Barbara, CA, and approved October 11, 2006 (sent for review July 8, 2006) New Zealand (NZ) has long been upheld as the archetypical Ma) dinosaur material (13) and isolated moa bones from marine example of a land where the biota evolved without nonvolant sediments up to 2.5 Ma (1, 14), the terrestrial record older than terrestrial mammals. Their absence before human arrival is mys- 1 Ma is extremely limited. Until now, there has been no direct terious, because NZ was still attached to East Antarctica in the Early evidence for the pre-Pleistocene presence in NZ of any of its Cretaceous when a variety of terrestrial mammals occupied the endemic vertebrate lineages, particularly any group of terrestrial adjacent Australian portion of Gondwana. -

Independence of the Endovestibular Potential in Homeotherms
Independence of the Endovestibular Potential in Homeotherms ROBERT S. SCHMIDT From the Department of Surgery (Otolaryngology), University of Chicago, Chicago ABS TR Ac T The endolymphatic potential was recorded from various vestibular parts of the labyrinth from which the cochlea (in the case of guinea pigs) or the cochlea, lagena, and sacculus (in the case of pigeons) had been removed. This endovesfibular potential of the isolated vestibule declined during anoxia and recovered after anoxia in the same manner as the endovestibular potential of the intact labyrinth. Its non-anoxic level was the same as in the intact laby- rinth; i.e., +5 to -[-8 mv in the pigeon and +2 to +5 mv in the guinea pig. It is, therefore, concluded that the endovestibular potential is independent of the cochlea, stria vascularis, and endocochlear potential. INTRODUCTION The endolymphatic potential discovered by B~k~sy (1) has been studied in both the cochlea (2) and vestibule (3, 4). This potential in the cochlea, the endocochlear potential (ECP), is about 80 mv positive in mammals and about 15 mv positive in birds (5). The potential in the vestibular parts of the labyrinth, the endovestibular potential (EVP), is much lower in homeotherms (4--6). Three assumptions regarding the EVP are quite common (4, 7, 8):--that nothing compared to the stria vascularis, the probable source of the ECP, is found in the vestibule; that the EVP results merely from spread of the ECP; and that the EVP is therefore of little interest or importance. These assumptions have very little theoretical or experimental foundation. -

Constraints on the Timescale of Animal Evolutionary History
Palaeontologia Electronica palaeo-electronica.org Constraints on the timescale of animal evolutionary history Michael J. Benton, Philip C.J. Donoghue, Robert J. Asher, Matt Friedman, Thomas J. Near, and Jakob Vinther ABSTRACT Dating the tree of life is a core endeavor in evolutionary biology. Rates of evolution are fundamental to nearly every evolutionary model and process. Rates need dates. There is much debate on the most appropriate and reasonable ways in which to date the tree of life, and recent work has highlighted some confusions and complexities that can be avoided. Whether phylogenetic trees are dated after they have been estab- lished, or as part of the process of tree finding, practitioners need to know which cali- brations to use. We emphasize the importance of identifying crown (not stem) fossils, levels of confidence in their attribution to the crown, current chronostratigraphic preci- sion, the primacy of the host geological formation and asymmetric confidence intervals. Here we present calibrations for 88 key nodes across the phylogeny of animals, rang- ing from the root of Metazoa to the last common ancestor of Homo sapiens. Close attention to detail is constantly required: for example, the classic bird-mammal date (base of crown Amniota) has often been given as 310-315 Ma; the 2014 international time scale indicates a minimum age of 318 Ma. Michael J. Benton. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Philip C.J. Donoghue. School of Earth Sciences, University of Bristol, Bristol, BS8 1RJ, U.K. [email protected] Robert J. -

Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma Tigrinum) Semicircular Canals
138 The Open Medical Informatics Journal, 2008, 2, 138-148 Open Access Mathematical Model of the Cupula-Endolymph System with Morphological Parameters for the Axolotl (Ambystoma tigrinum) Semicircular Canals Rosario Vega1, Vladimir V. Alexandrov2,3, Tamara B. Alexandrova1,3 and Enrique Soto*,1 1Instituto de Fisiología, Universidad Autónoma de Puebla, 2Facultad de Ciencias Físico Matemáticas, Universidad Autónoma de Puebla, 3 Lomonosov Moscow State University, Mexico Abstract: By combining mathematical methods with the morphological analysis of the semicircular canals of the axolotl (Ambystoma tigrinum), a system of differential equations describing the mechanical coupling in the semicircular canals was obtained. The coefficients of this system have an explicit physiological meaning that allows for the introduction of morphological and dynamical parameters directly into the differential equations. The cupula of the semicircular canals was modeled both as a piston and as a membrane (diaphragm like), and the duct canals as toroids with two main regions: i) the semicircular canal duct and, ii) a larger diameter region corresponding to the ampulla and the utricle. The endolymph motion was described by the Navier-Stokes equations. The analysis of the model demonstrated that cupular behavior dynamics under periodic stimulation is equivalent in both the piston and the membrane cupular models, thus a general model in which the detailed cupular structure is not relevant was derived. Keywords: Inner ear, vestibular, hair cell, transduction, sensory coding, physiology. 1. INTRODUCTION linear acceleration detectors, and the SCs as angular accel- eration detectors, notwithstanding that both sensory organs The processing of sensory information in the semicircular are based on a very similar sensory cell type. -

The Oldest Platypus and Its Bearing on Divergence Timing of the Platypus and Echidna Clades
The oldest platypus and its bearing on divergence timing of the platypus and echidna clades Timothy Rowe*†, Thomas H. Rich‡§, Patricia Vickers-Rich§, Mark Springer¶, and Michael O. Woodburneʈ *Jackson School of Geosciences, University of Texas, C1100, Austin, TX 78712; ‡Museum Victoria, PO Box 666, Melbourne, Victoria 3001, Australia; §School of Geosciences, PO Box 28E, Monash University, Victoria 3800, Australia; ¶Department of Biology, University of California, Riverside, CA 92521; and ʈDepartment of Geology, Museum of Northern Arizona, Flagstaff, AZ 86001 Edited by David B. Wake, University of California, Berkeley, CA, and approved October 31, 2007 (received for review July 7, 2007) Monotremes have left a poor fossil record, and paleontology has broadly affect our understanding of early mammalian history, been virtually mute during two decades of discussion about with special implications for molecular clock estimates of basal molecular clock estimates of the timing of divergence between the divergence times. platypus and echidna clades. We describe evidence from high- Monotremata today comprises five species that form two resolution x-ray computed tomography indicating that Teinolo- distinct clades (16). The echidna clade includes one short-beaked phos, an Early Cretaceous fossil from Australia’s Flat Rocks locality species (Tachyglossus aculeatus; Australia and surrounding is- (121–112.5 Ma), lies within the crown clade Monotremata, as a lands) and three long-beaked species (Zaglossus bruijni, Z. basal platypus. Strict molecular clock estimates of the divergence bartoni, and Z. attenboroughi, all from New Guinea). The between platypus and echidnas range from 17 to 80 Ma, but platypus clade includes only Ornithorhynchus anatinus (Austra- Teinolophos suggests that the two monotreme clades were al- lia, Tasmania). -

Mammals from the Mesozoic of Mongolia
Mammals from the Mesozoic of Mongolia Introduction and Simpson (1926) dcscrihed these as placental (eutherian) insectivores. 'l'he deltathcroids originally Mongolia produces one of the world's most extraordi- included with the insectivores, more recently have narily preserved assemblages of hlesozoic ma~nmals. t)een assigned to the Metatheria (Kielan-Jaworowska Unlike fossils at most Mesozoic sites, Inany of these and Nesov, 1990). For ahout 40 years these were the remains are skulls, and in some cases these are asso- only Mesozoic ~nanimalsknown from Mongolia. ciated with postcranial skeletons. Ry contrast, 'I'he next discoveries in Mongolia were made by the Mesozoic mammals at well-known sites in North Polish-Mongolian Palaeontological Expeditions America and other continents have produced less (1963-1971) initially led by Naydin Dovchin, then by complete material, usually incomplete jaws with den- Rinchen Barsbold on the Mongolian side, and Zofia titions, or isolated teeth. In addition to the rich Kielan-Jaworowska on the Polish side, Kazi~nierz samples of skulls and skeletons representing Late Koualski led the expedition in 1964. Late Cretaceous Cretaceous mam~nals,certain localities in Mongolia ma~nmalswere collected in three Gohi Desert regions: are also known for less well preserved, but important, Bayan Zag (Djadokhta Formation), Nenlegt and remains of Early Cretaceous mammals. The mammals Khulsan in the Nemegt Valley (Baruungoyot from hoth Early and Late Cretaceous intervals have Formation), and llcrmiin 'ISav, south-\vest of the increased our understanding of diversification and Neniegt Valley, in the Red beds of Hermiin 'rsav, morphologic variation in archaic mammals. which have heen regarded as a stratigraphic ecluivalent Potentially this new information has hearing on the of the Baruungoyot Formation (Gradzinslti r't crl., phylogenetic relationships among major branches of 1977). -

Fossils Importance Bibliography
GEOL 204 The Fossil Record Las Hoyas Spring 2020 Section 0101 Daniel Boyarsky, Noor Nabulsi, Alyssa Pryputniewicz & Elijah WeBB Fossils Geological Layout This Lagerstätten site has exceptionally preserved fossils. Some of these fossils include Microbial mats: responsible for the preservation of soft tissue in many fossils The iron structures that are rarely found in the fossil record, such as soft tissues, patterns of carbonate depositions, as a result from bacterial metabolism which covered the dinosaur's crest coloring, nerviation, and gut tracts (Buscalioni, A.D.). Below are some of the other animals increased the preservation of those soft tissues. Evidence of these mats comes from the studies that researchers have found fossils of. on microfacies and the fossils themselves. Albanerpeton (amphibian) Europejara (pterosaur) Crusafontia (mammal) Abruption: Notable in the formation, due to the presence of complex specimens. From studies on several different organisms it can be estimated that the burial of most entities was quick. Stagnation: Millions of years with the lack of external forces on the site created an untouched site of easily accessible fossils. 20 mm 35 cm 5 cm Fig. 1: Tamura, N. (Albanerpeton); Fig. 2: “Euro” Fig. 3: “Crusafontia” Venczel, M. Fig. 6: “Crusafontia” Http://Gomeslab.weebly.com/Uploads/1/0/9/2/10 Https://d1k5w7mbrh6vq5.Cloudfront.net/Images/ 9219815/Published/Img-1803- Cache/0d/34/7d/0d347d1e06f219283da2064b963 Importance 2.Jpg?1503670391, 5b420.Jpg,mages.app.goo.gl/qTGBW7NvY4X9v The Las Hoyas site has recorded a wide array of species of diverse plant and animal taxa. At images.app.goo.gl/C2zWPTU1rVD9VzoR7. 6HU8. present, the biodiversity count comprises 118 families and 201 species. -

Identification of a 275-Kd Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear G Richardson, Sylvain Bartolami, I Russell
Identification of a 275-kD Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear G Richardson, Sylvain Bartolami, I Russell To cite this version: G Richardson, Sylvain Bartolami, I Russell. Identification of a 275-kD Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear. Journal of Cell Biology, Rockefeller University Press, 1990, 110, 10.1083/jcb.110.4.1055. hal-02156410 HAL Id: hal-02156410 https://hal.archives-ouvertes.fr/hal-02156410 Submitted on 18 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Identification of a 275-kD Protein Associated with the Apical Surfaces of Sensory Hair Cells in the Avian Inner Ear G. P. Richardson, S. Bartolami, and I. J. Russell School of Biological Sciences, University of Sussex, Falmer, Brighton, United Kingdom Abstract. Immunological techniques have been used basilar papilla (an auditory organ) only the proximal to generate both polyclonal and monoclonal antibodies region of the stereocilia bundle nearest to the apical specific for the apical ends of sensory hair cells in the surface is stained. The monoclonal anti-hair cell anti- avian inner ear. -

Early Cretaceous Amphilestid ('Triconodont') Mammals from Mongolia
Early Cretaceous amphilestid ('triconodont') mammals from Mongolia ZOFIAKIELAN-JAWOROWSKA and DEMBERLYIN DASHZEVEG Kielan-Jaworowską Z. &Daslueveg, D. 1998. Early Cretaceous amphilestid (.tricono- dont') mammals from Mongotia. - Acta Pal.aeontol.ogicaPolonica,43,3, 413438. Asmall collection of ?Aptianor ?Albian amphilestid('triconodont') mammals consisting of incomplete dentaries and maxillae with teeth, from the Khoboor localiĘ Guchin Us counĘ in Mongolia, is described. Grchinodon Troftmov' 1978 is regarded a junior subjective synonym of GobiconodonTroftmov, 1978. Heavier wear of the molariforms M3 andM4than of themore anteriorone-M2 in Gobiconodonborissiaki gives indirect evidence formolariformreplacement in this taxon. The interlocking mechanismbetween lower molariforms n Gobiconodon is of the pattern seen in Kuchneotherium and Ttnodon. The ińterlocking mechanism and the type of occlusion ally Amphilestidae with Kuehneotheriidae, from which they differ in having lower molariforms with main cusps aligned and the dentary-squamosal jaw joint (double jaw joint in Kuehneotheńdae). The main cusps in upper molariforms M3-M5 of Gobiconodon, however, show incipient tńangular arrangement. The paper gives some support to Mills' idea on the therian affinities of the Amphilestidae, although it cannot be excluded that the characters that unite the two groups developed in parallel. Because of scanty material and arnbiguĘ we assign the Amphilestidae to order incertae sedis. Key words : Mammali4 .triconodonts', Amphilestidae, Kuehneotheriidae, Early Cretaceous, Mongolia. Zofia Kiel,an-Jaworowska [zkielnn@twarda,pan.pl], InsĘtut Paleobiologii PAN, ul. Twarda 5 I /5 5, PL-00-8 I 8 Warszawa, Poland. DemberĘin Dash7eveg, Geological Institute, Mongolian Academy of Sciences, Ulan Bator, Mongolia. Introduction Beliajeva et al. (1974) reportedthe discovery of Early Cretaceous mammals at the Khoboor locality (referred to also sometimes as Khovboor), in the Guchin Us Soinon (County), Gobi Desert, Mongolia. -
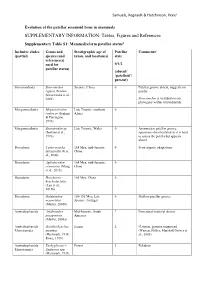
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels, Regnault & Hutchinson, PeerJ Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammaliaform patellar status$ Inclusive clades Genus and Stratigraphic age of Patellar Comments# (partial) species (and taxon, and location(s) state reference(s) used for 0/1/2 patellar status) (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic, China 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska et al., 2004) Sinoconodon is included on our phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji et China al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius (Meng China et al., 2015) Docodonta Docofossor 160 Mya, China 0 brachydactylus (Luo et al., 2015b) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Australosphenida Tachyglossus