Plant Species Associated with Some Asteraceae Plant and Edaphic Factor Effect Yasser A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proclaimed Plant Policy
Declared Plant Policy This policy relates to natural resources management under section 9(1)(d) of the Landscape South Australia Act 2019 (the Act), enabling co-ordinated implementation and promotion of sound management programs and practices for the use, development or protection of natural resources of the State. Specifically, this policy provides guidance on the use and management of natural resources relating to the prevention or control of impacts caused by pest species of plants that may have an adverse effect on the environment, primary production or the community, as per object s7(1)(f) of the Act. white weeping brooms (Retama raetam and Retama monosperma) The weeping white brooms are shrubs from the Mediterranean basin that are planted as ornamentals and encroach into native vegetation. Management Plan for White Weeping Brooms Outcome • Maintain the integrity of native vegetation by minimising invasion and impacts of white weeping broom, and preventing further spread. Objectives • Identification of the full extent of white weeping broom infestations in the active control areas. • High priority infestations of white weeping broom in the control areas controlled. • Increase awareness about the impacts of white weeping broom • No further spread of white weeping broom to currently uninfested areas. Best Practice Implementation • All regional landscape boards and Green Adelaide to enforce the prohibition on sale of white weeping broom. • Regional landscape boards in the active control areas to inspect, map and monitor infestations of white weeping broom in coastal and native vegetation. • Regional landscape boards in the active control areas to ensure high priority infestations on private or public land are controlled. -

Conserving Europe's Threatened Plants
Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation Conserving Europe’s threatened plants Progress towards Target 8 of the Global Strategy for Plant Conservation By Suzanne Sharrock and Meirion Jones May 2009 Recommended citation: Sharrock, S. and Jones, M., 2009. Conserving Europe’s threatened plants: Progress towards Target 8 of the Global Strategy for Plant Conservation Botanic Gardens Conservation International, Richmond, UK ISBN 978-1-905164-30-1 Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK Design: John Morgan, [email protected] Acknowledgements The work of establishing a consolidated list of threatened Photo credits European plants was first initiated by Hugh Synge who developed the original database on which this report is based. All images are credited to BGCI with the exceptions of: We are most grateful to Hugh for providing this database to page 5, Nikos Krigas; page 8. Christophe Libert; page 10, BGCI and advising on further development of the list. The Pawel Kos; page 12 (upper), Nikos Krigas; page 14: James exacting task of inputting data from national Red Lists was Hitchmough; page 16 (lower), Jože Bavcon; page 17 (upper), carried out by Chris Cockel and without his dedicated work, the Nkos Krigas; page 20 (upper), Anca Sarbu; page 21, Nikos list would not have been completed. Thank you for your efforts Krigas; page 22 (upper) Simon Williams; page 22 (lower), RBG Chris. We are grateful to all the members of the European Kew; page 23 (upper), Jo Packet; page 23 (lower), Sandrine Botanic Gardens Consortium and other colleagues from Europe Godefroid; page 24 (upper) Jože Bavcon; page 24 (lower), Frank who provided essential advice, guidance and supplementary Scumacher; page 25 (upper) Michael Burkart; page 25, (lower) information on the species included in the database. -

Wild Food Plants in Graecanic Communities in Calabria, Southern
Wild food plants in Graecanic communities in Calabria, Southern Italy - Ethnobotany, current role in Mediterranean diets, and antioxidant activity Thesis presented by Sabine M. Nebel for the degree of Doctor of Philosophy Centre for Pharmacognosy and Phytotherapy The School of Pharmacy University of London 2006 ’^OL OF " ProQuest Number: 10104805 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest 10104805 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 This thesis describes research conducted in the School of Pharmacy, University of London between 2002 and 2006 under the supervision of Prof. Michael Heinrich. I certify that the research described is original and that any parts of the work that have been conducted by collaboration are clearly indicated. I also certify that I have written all the text herein and have clearly indicated by suitable citation any part of this dissertation that has already appeared in publication. 7 / (^foC Signature Date Abstract Dietary patterns are changing rapidly all over the world. The body of available local food knowledge, which forms the basis of many local traditions, is decreasing dramatically. -

Distribution Agreement in Presenting This Thesis Or Dissertation As A
Distribution Agreement In presenting this thesis or dissertation as a partial fulfillment of the requirements for an advanced degree from Emory University, I hereby grant to Emory University and its agents the non-exclusive license to archive, make accessible, and display my thesis or dissertation in whole or in part in all forms of media, now or hereafter known, including display on the world wide web. I understand that I may select some access restrictions as part of the online submission of this thesis or dissertation. I retain all ownership rights to the copyright of the thesis or dissertation. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. Signature: _____________________________ ____3/31/16__________ Shelley Burian Date Flowers of Re: The floral origins and solar significance of rosettes in Egyptian art By Shelley Burian Master of Arts Art History _________________________________________ Rebecca Bailey, Ph.D., Advisor _________________________________________ Gay Robin, Ph.D., Committee Member _________________________________________ Walter Melion, Ph.D., Committee Member Accepted: _________________________________________ Lisa A. Tedesco, Ph.D. Dean of the James T. Laney School of Graduate Studies ___________________ Date Flowers of Re: The floral origins and solar significance of rosettes in Egyptian art By Shelley Burian B.A., First Honors, McGill University, 2011 An abstract of A thesis submitted to the Faculty of the James T. Laney School of Graduate Studies of Emory University in partial fulfillment of the requirements for the degree of Master of Arts In Art History 2016 Abstract Flowers of Re: The floral origins and solar significance of rosettes in Egyptian art By Shelley Burian Throughout the Pharaonic period in Egypt an image resembling a flower, called a rosette, was depicted on every type of art form from architecture to jewelry. -

IAPT Chromosome Data 28
TAXON 67 (6) • December 2018: 1235–1245 Marhold & Kučera (eds.) • IAPT chromosome data 28 IAPT CHROMOSOME DATA IAPT chromosome data 28 Edited by Karol Marhold & Jaromír Kučera DOI https://doi.org/10.12705/676.39 Julio Rubén Daviña & Ana Isabel Honfi* Chromosome numbers counted by L. Delgado and ploidy level estimated by B. Rojas-Andrés and N. López-González; collectors: Programa de Estudios Florísticos y Genética Vegetal, Instituto AA = Antonio Abad, AT = Andreas Tribsch, BR = Blanca Rojas- de Biología Subtropical CONICET-Universidad Nacional de Andrés, DGL = David Gutiérrez Larruscain, DP = Daniel Pinto, JASA Misiones, nodo Posadas, Rivadavia 2370, 3300 Posadas, Argentina = José Ángel Sánchez Agudo, JPG = Julio Peñas de Giles, LMC = * Author for correspondence: [email protected] Luz Mª Muñoz Centeno, MO = M. Montserrat Martínez-Ortega, MS = María Santos Vicente, NLG = Noemí López-González, NPG = This study was supported by Agencia Nacional de Promoción Nélida Padilla-García, SA = Santiago Andrés, SB = Sara Barrios, VL Científica y Técnica (ANPCyT) grant nos. PICT-2014-2218 and PICT- = Víctor Lucía, XG = Ximena Giráldez. 2016-1637, and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). This work has been supported by the Spanish Ministerio de Economía y Competitividad (projects CGL2009-07555, CGL2012- All materials CHN; collectors: D = J.R. Daviña, H = A.I. Honfi, 32574, Flora iberica VIII [CGL2008-02982-C03-02/CLI], Flora L = B. Leuenberger. iberica IX [CGL2011-28613-C03-03], Flora iberica X [CGL2014- 52787-C3-2-P]); the Spanish Ministerio de Ciencia e Innovación AMARYLLIDACEAE (Ph.D. grants to BR and NLG), and the University of Salamanca Habranthus barrosianus Hunz. -

NJ Native Plants - USDA
NJ Native Plants - USDA Scientific Name Common Name N/I Family Category National Wetland Indicator Status Thermopsis villosa Aaron's rod N Fabaceae Dicot Rubus depavitus Aberdeen dewberry N Rosaceae Dicot Artemisia absinthium absinthium I Asteraceae Dicot Aplectrum hyemale Adam and Eve N Orchidaceae Monocot FAC-, FACW Yucca filamentosa Adam's needle N Agavaceae Monocot Gentianella quinquefolia agueweed N Gentianaceae Dicot FAC, FACW- Rhamnus alnifolia alderleaf buckthorn N Rhamnaceae Dicot FACU, OBL Medicago sativa alfalfa I Fabaceae Dicot Ranunculus cymbalaria alkali buttercup N Ranunculaceae Dicot OBL Rubus allegheniensis Allegheny blackberry N Rosaceae Dicot UPL, FACW Hieracium paniculatum Allegheny hawkweed N Asteraceae Dicot Mimulus ringens Allegheny monkeyflower N Scrophulariaceae Dicot OBL Ranunculus allegheniensis Allegheny Mountain buttercup N Ranunculaceae Dicot FACU, FAC Prunus alleghaniensis Allegheny plum N Rosaceae Dicot UPL, NI Amelanchier laevis Allegheny serviceberry N Rosaceae Dicot Hylotelephium telephioides Allegheny stonecrop N Crassulaceae Dicot Adlumia fungosa allegheny vine N Fumariaceae Dicot Centaurea transalpina alpine knapweed N Asteraceae Dicot Potamogeton alpinus alpine pondweed N Potamogetonaceae Monocot OBL Viola labradorica alpine violet N Violaceae Dicot FAC Trifolium hybridum alsike clover I Fabaceae Dicot FACU-, FAC Cornus alternifolia alternateleaf dogwood N Cornaceae Dicot Strophostyles helvola amberique-bean N Fabaceae Dicot Puccinellia americana American alkaligrass N Poaceae Monocot Heuchera americana -

Significance Important of Fruit Character for Some Asteraceae Species in Identification and Differentiation Level Dalia Goda Ibrahim Gabr*
Haya: The Saudi Journal of Life Sciences Abbreviated Key Title: Haya Saudi J Life Sci ISSN 2415-623X (Print) |ISSN 2415-6221 (Online) Scholars Middle East Publishers, Dubai, United Arab Emirates Journal homepage: http://scholarsmepub.com/haya/ Original Research Article Significance Important of Fruit Character for Some Asteraceae Species in Identification and Differentiation Level Dalia Goda Ibrahim Gabr* Department of Basic Science, Faculty of Education, Imam Abdulrahman Bin Faisal University, Saudi Arabia 7322 Amir Ib Fahira – Ar Rawdah, Unit No. 3, Ad /Dammam 32256 – 3210, Kingdom of Saudi Arabia DOI: 10.36348/SJLS.2019.v04i08.003 | Received: 21.09.2019 | Accepted: 28.09.2019 | Published: 30.09.2019 *Corresponding author: Dalia Goda Ibrahim Gabr Abstract Achene and pappus macro and micro-morphological characters for 10 species belong to two sub-family of Asteraceae from eastern region of Saudi Arabia to evaluate the application of this character in the identification and differentiation level by using light microscope (LM) and scanning electron microscope (SEM). The achene morphological characters as, size, shape, color, texture, ridges, base, achene coat and pappus characters are done. The achene coat pattern sculpture recorded 6 types; striate, tuberculate, granulate, sulcate, irregular reticulate and reticulate-rugose, the main types were reticulate. The pappus type’s recorded three types; scabrous barbellate bristles, scabrous subulate scales free and capillary barbelllate, the main types were scabrous barbellate bristles found in seven studied species. The result for this study supports the use of achene morphological characters for identification and differentiates of different related species but cannot be used for taxonomical levels for the different tribes. -

J.F. Veldkamp (Continued from Page 104)
BIBLIOGRAPHY: BRYOPHYTES 165 XVI. Bibliography J.F. Veldkamp (continued from page 104) * Books have been marked with an asterisk. BRYOPHYTES AKIYAMA, H. 1988. Studies onLeucodon (Leucodontaceae, Musci)and related genera in East Asia III. Notes on the systematic position of Pterogonium. Acta Phytotax. Geo- bot. 39: 73-82, 4 fig. — To Isobryales near Anomodon. ASAKAWA, Y. 1988. Chemicalevolution of mono- and sesquiterpenoids ofliverworts. J. Hattori Bot. Lab. 64: 97-108, 16 fig. BISCHLER, H. 1989. MarchantiaL.: subg. Chlamidium (Nees) Bischl. sect. Papillatae Bischl. sect. nov. en Asie et en Ocianie. Cryptog., Bryol. Lichenol. 10: 61-79, 9 fig, 3 tab. (In French, Engl. summ.). — Marchantia emarginata group, 2 species, 5 sub- species. - — 1988. Marchantiapaleacea Bertol. Karyotype analysis. Beih. Nova Hedw. 90 (1988) 95-100, 2 fig, 1 tab. — 1988. Relationships in the order Marchantiales (Hepaticae). J. Hattori Bot. Lab. 64: 47-57, 3 tab. BUCK, W.R. 1988. Another view ofthe familial delimitationofthe Hookeriales. J. Hattori Bot. Lab. 64: 29-36,1 fig. — 5 families; key; descriptions. CAP, T. & C. GAO. 1988. Studies ofChinese bryophytes. (2). Trematodon Michx. (Mus- ci, Dicranaceae). J. Hattori Bot. Lab. 65: 323-334, 6 fig, 1 tab. — 2 species, 1 Male- sian; descriptions. CATCHESIDE, D.G. 1988. The mosses of the Northern territory, Australia. J. Adelaide Bot. Gard. 11: 1-17, 4 — 95 54 new records, fig. species, keys to some genera. CHANDRA, V., et al. 1987. Calobryales: Distribution andphytogeographical discussion. Geophytology 17: 227-232, 1 map. * EDDY, A. 1988. A handbook ofMalesian mosses. 1. Sphagnales to Dicranales. iii, 204 165 British London. ISBN 0-567-01038-7. -

Genetic Diversity and Evolution in Lactuca L. (Asteraceae)
Genetic diversity and evolution in Lactuca L. (Asteraceae) from phylogeny to molecular breeding Zhen Wei Thesis committee Promotor Prof. Dr M.E. Schranz Professor of Biosystematics Wageningen University Other members Prof. Dr P.C. Struik, Wageningen University Dr N. Kilian, Free University of Berlin, Germany Dr R. van Treuren, Wageningen University Dr M.J.W. Jeuken, Wageningen University This research was conducted under the auspices of the Graduate School of Experimental Plant Sciences. Genetic diversity and evolution in Lactuca L. (Asteraceae) from phylogeny to molecular breeding Zhen Wei Thesis submitted in fulfilment of the requirements for the degree of doctor at Wageningen University by the authority of the Rector Magnificus Prof. Dr A.P.J. Mol, in the presence of the Thesis Committee appointed by the Academic Board to be defended in public on Monday 25 January 2016 at 1.30 p.m. in the Aula. Zhen Wei Genetic diversity and evolution in Lactuca L. (Asteraceae) - from phylogeny to molecular breeding, 210 pages. PhD thesis, Wageningen University, Wageningen, NL (2016) With references, with summary in Dutch and English ISBN 978-94-6257-614-8 Contents Chapter 1 General introduction 7 Chapter 2 Phylogenetic relationships within Lactuca L. (Asteraceae), including African species, based on chloroplast DNA sequence comparisons* 31 Chapter 3 Phylogenetic analysis of Lactuca L. and closely related genera (Asteraceae), using complete chloroplast genomes and nuclear rDNA sequences 99 Chapter 4 A mixed model QTL analysis for salt tolerance in -
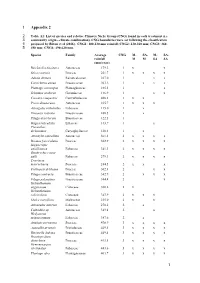
1 Appendix 2 1 2 3
1 Appendix 2 2 Table A1: List of species and relative Climatic Niche Group (CNG) found in each treatment (i.e. 3 community origin - climate combination). CNG boundaries were set following the classification 4 proposed by Bilton et al (2016). CNG1: 100-230 mm rainfall; CNG2: 230-360 mm; CNG3: 360- 5 490 mm; CNG4: 490-620 mm. Species Family Average CNG M- SA- M- SA- rainfall M M SA SA (mm/year) Reichardia tingitana Asteraceae 179.3 1 x x Stipa capensis Poaceae 221.7 1 x x x x Adonis dentata Ranunculaceae 227.0 1 x x Carrichtera annua Brassicaceae 203.2 1 x x x Plantago coronopus Plantaginaceae 185.5 1 x Schismus arabicus Geraniaceae 146.9 1 x x Cuscuta campestris Convolvulaceae 488.1 1 x x Picris damascena Asteraceae 165.7 1 x x x Astragalus tribuloides Fabaceae 115.0 1 x Erucaria rostrata Brassicaceae 168.5 1 x Filago desertorum Brassicaceae 122.5 1 Gagea reticultata Liliaceae 143.7 1 x Pteranthus dichotomus Caryophyllaceae 120.3 1 x Atractylis cancellata Asteraceae 361.1 2 x x x x Bromus fasciculatus Poaceae 302.9 2 x x x x Hippocrepis unisiliquosa Fabaceae 341.3 2 x x x x Onobrychis crista- galli Fabaceae 279.3 2 x x x x Trisetaria macrochaeta Poaceae 244.5 2 x x x Crithopsis delileana Poaceae 302.1 2 x x Filago contracta Brassicaceae 342.9 2 x x x Filago palaestina Brassicaceae 344.4 2 x Helianthemum aegytiacum Cistaceae 360.2 2 x Helianthemum salicifolium Cistaceae 347.9 2 x x x Malva parviflora Malvaceae 235.0 2 x x Astragalus asterias Fabaceae 278.2 2 x Calendula sp. -

Native Species
Birdlife Australia Gluepot Reserve PLANT SPECIES LIST These are species recorded by various observers. Species in bold have been vouchered. The list is being continually updated NATIVE SPECIES Species name Common name Acacia acanthoclada Harrow Wattle Acacia aneura Mulga Acacia brachybotrya Grey Mulga Acacia colletioides Wait a While Acacia hakeoides Hakea leaved Wattle Acacia halliana Hall’s Wattle Acacia ligulata Sandhill Wattle Acacia nyssophylla Prickly Wattle Acacia oswaldii Boomerang Bush Acacia rigens Needle Wattle Acacia sclerophylla var. sclerophylla Hard Leaved Wattle Acacia wilhelmiana Wilhelm’s Wattle Actinobole uliginosum Flannel Cudweed Alectryon oleifolius ssp. canescens Bullock Bush Amphipogon caricinus Long Grey Beard Grass Amyema miquelii Box Mistletoe Amyema miraculosa ssp. boormanii Fleshy Mistletoe Amyema preissii Wire Leaved Acacia Mistletoe Angianthus tomentosus Hairy Cup Flower Atriplex acutibractea Pointed Salt Bush Atriplex rhagodioides Spade Leaved Salt Bush Atriplex stipitata Bitter Salt Bush Atriplex vesicaria Bladder Salt Bush Austrodanthonia caespitosa Wallaby Grass Austrodanthonia pilosa Wallaby Grass Austrostipa elegantissima Elegant Spear Grass Austrostipa hemipogon Half Beard Spear grass Austrostipa nitida Balcarra Spear grass Austrostipa scabra ssp. falcata Rough Spear Grass Austrostipa scabra ssp. scabra Rough Spear Grass Austrostipa tuckeri Tucker’s Spear grass Baeckea crassifolia Desert Baeckea Baeckea ericaea Mat baeckea Bertya tasmanica ssp vestita Mitchell’s Bertya Beyeria lechenaultii Mallefowl -

Germination Ecology of Two Indigenous Range Grasses Lasiurus Scindicus and Panicum Turgidum Naeema Sultan Abdullah Al-Shamisi
United Arab Emirates University Scholarworks@UAEU Theses Electronic Theses and Dissertations 2009 Germination Ecology of Two Indigenous Range Grasses Lasiurus scindicus and Panicum turgidum Naeema Sultan Abdullah Al-Shamisi Follow this and additional works at: https://scholarworks.uaeu.ac.ae/all_theses Part of the Environmental Sciences Commons Recommended Citation Abdullah Al-Shamisi, Naeema Sultan, "Germination Ecology of Two Indigenous Range Grasses Lasiurus scindicus and Panicum turgidum" (2009). Theses. 605. https://scholarworks.uaeu.ac.ae/all_theses/605 This Thesis is brought to you for free and open access by the Electronic Theses and Dissertations at Scholarworks@UAEU. It has been accepted for inclusion in Theses by an authorized administrator of Scholarworks@UAEU. For more information, please contact [email protected]. United Arab Emirates University Deanship of Graduate Studies M.Sc. Program in Environmental Sciences GERMINATION ECOLOGY OF TWO INDIGENOUS RANGE GRASSES LASIURUS SCINDICUS AND PANICUM TURGIDUM By Naeema Sultan Abdullah AI-Shamisi A thesis submitted to United Arab Emirates University In Partial Fulfillment of the Requirements For the Degree of M.Sc. in Environmental Sciences 2009 United Arab Emirates University Deanship of Graduate Studies M.Sc. Program in Environmental Sciences GERMINA TION ECOLOGY OF TWO INDIGENOUS RANGE GRASSES LASlUR US SClNDICUS AND PANICUM TURGIDUM By Naeema Sultan Abdullah Al-Shamisi A thesis Submitted to United Arab Emirates University In Partial Fulfillment of the Requirements For the Degree of M.Sc. in Environmental Sciences Supervisors Fatima Mahmoud AI-Ansari Ali Ali EI-Keblawy Associate Professor Associate Professor Department of Biology Department of Biology College of Science College of Science UAE University UAE University 2009 The Thc, i of aeema ultan Alshamisi for the Degrec of Ma ter or nmental i appro ed.