Primulina Cardaminifolia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plateau Mountain Plant List
Plateau ALBERTA WILDERNESS Mountain – Plant list ASSOCIATION Plants of Plateau Mountain Ecological Reserve (Plateau Mountain Ecological Reserve Management Plan – Alberta Environment) Alpine Anemone Anemone drummondii Drummond's Rock Cress Alpine Arnica Arnica angustifolia Drummond's Rush Alpine Bistort Polygonum viviparum Dwarf Birch Betula glandulosa Alpine Blue Grass Poa alpina Dwarf Bitter root Alpine Everlasting Antennaria sp. Dwarf Hawk's Beard Alpine Fleabane Erigeron pallens Dwarf Sawwort Saussurea nuda Alpine Forget-me not Myosotis alpestris Dwarf saw-wort Saussurea nuda Alpine Goldenrod Solidago multiradianta Dwarf Scouring-rush Equisetum scirpoides Alpine Hawkweed Early Blue Grass Alpine Milk-vetch Astragalus alpinus Early Blue Violet Viola adunca Alpine mouse-eared chickweed Cerastium beeringianum Early Cinquefoil Potertilla concinna Alpine Speedwell Veronica alpina Elephant's head Pedicularis groenlandica Alpine speedwell Veronica alpina Elephant's-head Lousewort Pedicualaris groenlandica Alpine Timothy Elgelmann Spruce Picea engelmanii American Vetch Entire-leaved Groundsel Androsace Androsace chamaejasme Everlasting Antennaria luzuloides Arctic Aster False Dandelion Agoseris aurantiaca Arctic Blue Glass Felwort Gentianela amarella Arctic Butterweed Few-flowered Milk-vetch Astragalus sp. Balsam Groundsel Few-seeded Whitlow-grass Draba oligosperma Balsam Poplar Populus balsamifera Field Chickweed Stellaria sp. Barratt's Willow Fireweed Locoweed Oxytropis sp. Bearberry Arctostaphylos uva-ursi Flame-colored Lousewort Pedicularis -
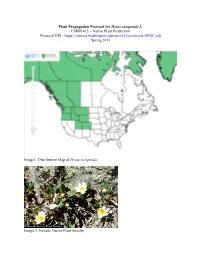
Plant Propagation Protocol for Dryas Octopetala L. ESRM 412 – Native Plant Production Protocol URL
Plant Propagation Protocol for Dryas octopetala L. ESRM 412 – Native Plant Production Protocol URL: https://courses.washington.edu/esrm412/protocols/DROC.pdf Spring 2015 Image1. Distribution Map of Dryas octopetala Image 2. Nevada Native Plant Society TAXONOMY Plant Family [3] Scientific Name Rosaceae Common Name Rose Family Species Scientific Name Scientific Name Dryas octopetala L. [3] Varieties * Dryas octopetala var. angustifolia C.L. Hitchc. [3] Dryas octopetala var. hookeriana (Juz) Hulten Sub-species Dryas octopetala f. argentea (Blytt) Hulten * Dryas octopetala subsp. alaskensis (Porsild) Hulten [3] Cultivar Common Synonym(s) Dradetum octopetalae Keiner Common Name(s) White mountain-avens, Eightpetal mountain-avens, Mountain dryas Species Code (as per USDA Plants DROC database) GENERAL INFORMATION Geographical range Alaska, Washington, Oregon, Colorado Alpine regions in the Pacific Northwest N. Cascades, and Rocky Mountain ranges Ecological distribution Mid-montane to Alpine zone Climate and elevation range Elevation: 3,500 m [6] 100 m and less Climate: sites with low snow cover on calcareous or basic soils [6] Local habitat and abundance Full sun Dry, well-drained, sandy or gravelly soils Spreads rapidly [6] Dominant or co-dominant species within its range [6] Associated species: Dwarf willow Plant strategy type / successional Nitrogen fixer: forms association with Frankia [5] stage Colonizer of barren slopes at high elevations [5] Plant characteristics Forb/herb, Shrub, Subshrub Perrennial Forms mats up to 3 ft. wide and 8 in. tall. [1] 1 cream or white flower at the end of each 2-8 inch leafless flower stalk. [2] Flowers bloom June-July Summer fruits fluffy and feathery Seeds are wind-dispersed [7] Leaves are oval-shaped, leathery with rounded teeth and a white underside. -

Dryas Octopetala Subsp. Alaskensis: Potential for a Hardy, Novel Flower
Dryas octopetala subsp. alaskensis: Potential for a Hardy, Novel Flower Eamon Spanier-Scanlon, Student of Plant Science University of Minnesota, St. Paul, MN 5/13/2019 Executive Summary Dryas octopetala, Mountain Avens, is a new sprawling variety of ground cover which may become available in northern climates near you. This extremely cold-tolerant plant will spread easily in your garden or within a larger pot with other plants. With petite white flowers blooming late in the spring, and placed gingerly upon thin red stalks, their beauty persists throughout the summer. This perennial ground cover will return year after year, regardless of the harshest winters. Purchase a few and watch as they creep around your yard, or simply buy one and propagate your own, with ease, through cuttings. The northern consumer may often feel left out by the lack of perennial flowering plants, but with this new species you can soon become the envy of your friends residing in the south. Minimal input is required for these beautiful white flowers to flourish in your domain. Pick up your own in a local floral retailer and fall in love over and over, each spring, as they bound back to life. I. Introduction A. Species The distribution of the genus Dryas is worldwide; they are often found in harsh areas such as arctic and tundra regions (Springer et al., 2019). Dryas octopetala is currently on the market as a decorative, flowering plant, although subsp. alaskensis is not. It can be purchased as a seed from multiple vendors, coming in such names as “Mountain Evans,” available through GeoSeed company (https://www.geoseed.com). -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

Gesneriads First Quarter 2018
GesThe Journal forn Gesneriade Growersria ds Volume 68 ~ Number 1 First Quarter 2018 Return to Table of Contents RETURN TO TABLE OF CONTENTS The Journal for Gesneriad Growers Volume 68 ~ Number 1 Gesneriads First Quarter 2018 FEATURES DEPARTMENTS 5 Saintpaulia, the NEW Streptocarpus 3 Message from the President Winston Goretsky Julie Mavity-Hudson 9 Style Guide for Writers 4 From The Editor Jeanne Katzenstein Peter Shalit 10 Gesneriads at the Liuzhou Arts Center 18 Gesneriad Registrations Wallace Wells Irina Nicholson 24 Flower Show Awards 42 Changes to Hybrid Seed List 4Q17 Paul Susi Gussie Farrice 25 Gesneriads POP in New England! 46 Coming Events Maureen Pratt Ray Coyle and Karyn Cichocki 28 62nd Annual Convention of The 47 Flower Show Roundup Gesneriad Society 51 Back to Basics: Gesneriad Crafts 37 Convention Speakers Dale Martens Dee Stewart 55 Seed Fund – Species 39 Petrocosmeas in the United Kingdom Carolyn Ripps Razvan Chisu 61 Information about The Gesneriad 43 Gasteranthus herbaceus – A white- Society, Inc. flowered Gasteranthus from the northern Andes Dale Martens with John L. Clark Cover Eucodonia ‘Adele’ grown by Eileen McGrath Back Cover and exhibited at the New York State African Petrocosmea ‘Stone Amethyst’, hybridized, Violet Convention Show, October 2017. grown, and photographed by Andy Kuang. Photo: Bob Clark See New Registrations article, page 18. Editor Business Manager The Gesneriad Society, Inc. Peter Shalit Michael A. Riley The objects of The Gesneriad [email protected] [email protected] Society are to afford -

Palaeoenvironment in North-Western Romania During the Last 15,000 Years
Palaeoenvironment in north-western Romania during the last 15,000 years Angelica Feurdean Dedicated to Ovidiu Feurdean Avhandling i Kvartärgeologi Thesis in Quaternary Geology No. 3 Department of Physical Geography and Quaternary Geology, Stockholm University 2004 1 ISBN 2 Palaeoenvironment in north-western Romania during the last 15,000 years by Angelica Feurdean Department of Physical Geography and Quaternary Geology, Stockholm University, SE-106 91 Stockholm This thesis is based on work carried out as Ph.D. stu- Appendix III: Feurdean A. (in press). Holocene forest dent at the Department of Paleontology, Faculty of Biol- dynamics in north-western Romania. The Holocene. ogy and Geology, Babes-Bolyai University, Cluj-Napoca, Romania between Oct. 1998 and Sept. 2002 and later as Appendix IV: Feurdean A. & Bennike O. Late Quater- Ph.D. student in Quaternary Geology at the Department nary palaeocological and paleoclimatological reconstruc- of Physical Geography and Quaternary Geology, tion in the Gutaiului Mountains, NW Romania. Manu- Stockholm University 2002-2004. The thesis consists of script submitted to Journal of Quaternary Science. four papers and a synthesis. The four papers are listed below and presented in Appendices I-IV. Two of the pa- Fieldwork in Romania has been jointly performed with pers have been published (I, II), one is in press (III) and Barbara Wohlfarth and Leif Björkman (Preluca Tiganului, the fourth has been submitted (IV). The thesis summary Steregoiu, Izvoare) and with Bogdan Onac (Creasta presents an account of earlier pollenstratigraphic work Cocosului). I am responsible for the lithostratigraphic done in Romania and the discussion focuses on tree description of all sediment and peat cores, for sub-sam- dynamics during the Lateglacial and Holocene, based pling and laboratory preparation. -

Northwest Plant Names and Symbols for Ecosystem Inventory and Analysis Fourth Edition
USDA Forest Service General Technical Report PNW-46 1976 NORTHWEST PLANT NAMES AND SYMBOLS FOR ECOSYSTEM INVENTORY AND ANALYSIS FOURTH EDITION PACIFIC NORTHWEST FOREST AND RANGE EXPERIMENT STATION U.S. DEPARTMENT OF AGRICULTURE FOREST SERVICE PORTLAND, OREGON This file was created by scanning the printed publication. Text errors identified by the software have been corrected; however, some errors may remain. CONTENTS Page . INTRODUCTION TO FOURTH EDITION ....... 1 Features and Additions. ......... 1 Inquiries ................ 2 History of Plant Code Development .... 3 MASTER LIST OF SPECIES AND SYMBOLS ..... 5 Grasses.. ............... 7 Grasslike Plants. ............ 29 Forbs.. ................ 43 Shrubs. .................203 Trees. .................225 ABSTRACT LIST OF SYNONYMS ..............233 This paper is basicafly'an alpha code and name 1 isting of forest and rangeland grasses, sedges, LIST OF SOIL SURFACE ITEMS .........261 rushes, forbs, shrubs, and trees of Oregon, Wash- ington, and Idaho. The code expedites recording of vegetation inventory data and is especially useful to those processing their data by contem- porary computer systems. Editorial and secretarial personnel will find the name and authorship lists i ' to be handy desk references. KEYWORDS: Plant nomenclature, vegetation survey, I Oregon, Washington, Idaho. G. A. GARRISON and J. M. SKOVLIN are Assistant Director and Project Leader, respectively, of Paci fic Northwest Forest and Range Experiment Station; C. E. POULTON is Director, Range and Resource Ecology Applications of Earth Sate1 1 ite Corporation; and A. H. WINWARD is Professor of Range Management at Oregon State University . and a fifth letter also appears in those instances where a varietal name is appended to the genus and INTRODUCTION species. (3) Some genera symbols consist of four letters or less, e.g., ACER, AIM, GEUM, IRIS, POA, TO FOURTH EDITION RHUS, ROSA. -

Primulina Serrulata Sp
A peer-reviewed open-access journal PhytoKeys 132: 11–18 (2019) Primulina serrulata sp. nov. from China 11 doi: 10.3897/phytokeys.132.36717 RESEARCH ARTICLE http://phytokeys.pensoft.net Launched to accelerate biodiversity research Primulina serrulata (Gesneriaceae), a new species from southeastern Guizhou, China Hong Jiang1, Tan Deng1, Xin-Yun Lv1, Ren-Bo Zhang1, Fang Wen2,3,4 1 Department of Biology, Zunyi Normal College, Zunyi, Guizhou 563002, China 2 Guangxi Key Laboratory of Plant Conservation and Restoration Ecology in Karst Terrain, Guilin Botanical Garden, Guangxi Institute of Botany, Guangxi Zhuangzu Autonomous Region and Chinese Academy of Sciences, Guilin 541006, China 3 Gesneriad Conservation Center of China, Guilin Botanical Garden, Chinese Academy of Sciences, Guilin 541006, China 4 Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China Corresponding author: Fang Wen ([email protected]) Academic editor: E. Fischer | Received 3 June 2019 | Accepted 30 August 2019 | Published 19 September 2019 Citation: Jiang H, Deng T, Lv X-X, Zhang R-B, Wen F (2019) Primulina serrulata (Gesneriaceae), a new species from southeastern Guizhou, China. PhytoKeys 132: 11–18. https://doi.org/10.3897/phytokeys.132.36717 Abstract Primulina serrulata R.B.Zhang & F. Wen, a new species from a limestone area in southeastern Guizhou, China, is described and illustrated here. The new species is morphologically related toP. fimbrisepala (Hand.-Mazz.) Y.Z.Wang. We examined the morphological differences between these congeners and pro- vide illustrations and photographs of this new species in this paper. Keywords Flora of Guizhou, Karst region, New taxa, Primulina fimbrisepala Introduction The formerly monotypic genus,Primulina Hance, has become the largest genus in the subfamily Didymocarpoideae of Gesneriaceae in China (Weber et al. -

Dryas Octopetala L
Dryas octopetala L. Mountain Avens Dryas octopetala is a long-lived dwarf-shrub. It has large white flowers with 8(-10) petals and leaves with a distinctive crenate margin. These are dark-glossy-green above but have a dense covering of white hairs on the underside. It is associated with open, base-rich calcareous heath and limestone grassland habitats on thin infertile soils, and also with rock-ledge communities on limestone and mica-schist. It is locally abundant in parts of Scotland and western Ireland but is rare in England and Wales. It is assessed as of Least Concern in Great Britain, but as Vulnerable in England and Endangered in Wales. ©Kevin Walker IDENTIFICATION variety or subspecies by British botanists (e.g. Stace 2010). Dryas octopetala is a prostrate dwarf shrub with large white flowers that have 8(-10) petals and numerous yellow carpels. HABITATS The fruit has a distinctive long, persistent and feathery style. In the British Isles D. octopetala is a very localised plant of The leaves are dark glossy green above with a crenate margin open base-rich habitats, mainly in the uplands, but also at and are densely white-tomentose on the underside. sea-level in north and west Scotland and Ireland (Elkington 1971). It grows on substrates derived from basic rocks SIMILAR SPECIES (limestones, basalts, mica-schists), usually where the soil is not too deep and drainage is very good. Dryas octopetala is unmistakable in all stages of growth and is unlikely to be confused with any other British or Irish In Britain ‘Dryas-heath’, represented by NVC CG13 Dryas species. -

Lquat Arctic Alpine Plants
The Late -Quaternary History of Arctic and Alpine Plants and their future in a warming world Hilary H. Birks University of Bergen How do we reconstruct the history of flora and vegetation? Where do we find our evidence and how do we interpret it? The history of the history What were past climates like and how did they change? How did plants survive climate change? The most recent glacial climate – the Younger Dryas How did arctic and alpine plants react to Holocene warming? What does the future hold for Arctic and Alpine plants? 1 1. Evidence of past flora and its changes Fossils • Pollen - microscopic • Macrofossils – can be seen with naked eye. Seeds, fruits, leaves, etc. Molecular DNA analyses of living arctic alpines can complement the fossil record. Find different populations in space today and deduce past migrations Fossil DNA extraction from sediments or plant remains is becoming increasingly sophisticated Pollen grains and spores • Walls are sporopollenin, very resistant to decay • Preserve well in anaerobic environments, e.g. lake sediments, peats • Can be extracted from the sediment matrix using chemicals to remove the organic and inorganic sediment components • Counted under a high-power microscope • Frequent enough to allow percentage calculations of abundance 2 BUT - in glacial and late-glacial environments: • Wind-dispersed pollen types dominate the assemblages (grasses, sedges, Artemisia ) • Arctic and alpine herbs generally produce rather little pollen • They are frequently insect pollinated • In landscapes with plants -

Potentilla Fruticosa L
Potentilla fruticosa L. Shrubby Cinquefoil Potentilla fruticosa is a deciduous shrub with pinnate leaves and attractive yellow flowers with petals longer than sepals. Although cultivars of this species are widely planted throughout the British Isles P. fruticosa is rare as a native. It is restricted to Upper Teesdale, where it is most often found in base-rich streamside vegetation, the Cumbrian Fells, where it occurs as a montane plant of damp rock ledges, and the Burren region of western Ireland, where it is associated with turloughs, scrub and fen meadow. It is assessed as Near Threatened in Great Britain due to its restricted distribution and recent decline. © Jer emy Roberts IDENTIFICATION material. Bare winter stems often trap flood debris, creating a characteristically scruffy appearance. Potentilla fruticosa is a deciduous flowering shrub typically attaining about a metre in height, although plants may be very much smaller. It is simple to identify when in flower: SIMILAR SPECIES excluding the prostrate Helianthemum species, it is the only Immature deciduous shrubs of other species may cause British native shrub bearing regular, showy yellow flowers problems in winter. P. fruticosa typically combines quite with petals (6-16 mm) much longer than the sepals (Stace, dense branching from multiple, clustered stems with a fine, 2010). The pinnate leaves are distinctive (although they whippy branch form. The remains of the previous year's sometimes appear palmate in small specimens), allowing stipules can be seen on most nodes and this confirms the summer recognition of small, grazed or layering plants only a plant. P. fruticosa cultivars (e.g. -
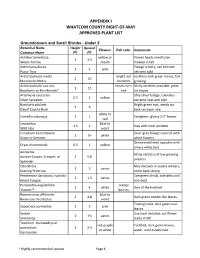
Whatcom County's Approved Plant List
APPENDIX I WHATCOM COUNTY RIGHT-OF-WAY APPROVED PLANT LIST Groundcovers and Small Shrubs - Under 2' Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Achillea tomentosa yellow or Flower heads need to be 1 3-5 Wooly Yarrow cream mowed in fall Antennaria dioica Foliage is furry, can tolerate 1 2 pink Pussy Toes extreme cold Arctostaphylos media bright red Leathery dark green leaves, fast 2 10 Manzanita Media branches growing Arctostaphylos uva ursi leaves turn Many varieties available, great 1 15 Bearberry or Kinnikinnick* red for slopes Artemesia caucasica Silky silver foliage, tolerates 0.5 2 yellow Silver Spreader extreme heat and cold Baccharis pilularis Bright green mat, needs cut 2 6 Dwarf Coyote Bush back once per year white to Camellia sasanqua 1 1 Evergreen, glossy 2-3" leaves red Ceanothus blue to 1.5 2 Stay with local varieties Wild Lilac violet Cerastium tomentosum Silver gray foliage covered with 1 5+ white Snow-in-Summer white flowers Ornamental seed capsules with Dryas drummondii 0.5 1 yellow silvery white tails Juniperus Many varieties of low growing Juniper-Carpet, Creeper, or 2 5-8 junipers Spreader Oenothera May die back in severe winters, 1 2 varies Evening Primrose come back strong Penstemon davidsonii, rupicola Evergreen shrub, tolerates cold 2 1.5 varies Beard Tongue and heat Pyracantha augustifolia orange 1 4 white One of the hardiest 'Gnome'* berries Rosmariunus officinalis blue to 2 4-8 Dark green needle-like leaves Rosemary 'Prostratus' violet Trailing habit, dark green oval Saponaria ocymoides 1 3 pink leaves Sedum Use local varieties, cut flower 2 1-5 varies Stonecrop stalks in fall Teucrium chamaedrys or red-purple Toothed, dark green leaves, postratium 1 2-3 or white water until established Germander * Highly-recommended species Page 1 Thymus white to 2 1-2 Many varieties Thyme purple Shrubs Botanical Name Height Spread Flowers Fall color Comments Common Name (ft) (ft) Arctostaphylos bright red Shiny dark green leaves turn 2-15 20 varies Manzanita berries maroon in fall.