Prevalence of the Fungal Pathogen Batrachochytrium Dendrobatidis In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Español, Que Estarán Disponibles En Dades De Conservación (
AArk Boletin Informativo BoletinNúmero 35, Junio 2016 amphibian ark Informativo Manteniendo las especies amenazadas de anfibios a flote Número 35, Junio 2016 En esta edición... Becas Semilla del Arca de Anfibios 2016 ......... 2 Cursos de Manejo de Salamandrass ............... 4 ® Segundo “Avance” del Arca de los Anfibios ...... 6 Un programa de conservación ex situ de la Rana de Collar Merideña.................................. 7 Videos tutoriales de la Evaluación de las Necesidades de Conservación ........................ 8 Amphibian Advocates - Jen Stabile, Directora de Conservación e Investigación, Zoológico de San Antonio ................................................. 9 Premio Futuro de la Naturaleza...................... 10 Curso de entrenamiento en Manejo y Conservación de Anfibios en Portugal............ 11 Documentos recientes de manejo en el sitio web de AArk ................................................... 13 “La Roca” ayuda a promover la conservación de la Rana del Lago Titicaca .......................... 14 Salvando la Rana del lagoTiticaca ................. 15 Reconocimientos de los donantes, enero- junio 2016 ....................................................... 16 Amphibian Ark c/o Conservation Breeding Specialist Group 12101 Johnny Cake Ridge Road Apple Valley MN 55124-8151 USA www.amphibianark.org Teléfono: +1 952 997 9800 Fax: +1 952 997 9803 World Association of Zoos and Aquariums | WAZA 1 www.amphibianark.org United for Conservation AArk Boletin Informativo Número 35, Junio 2016 Becas Semilla del Arca de Anfibios 2016 Kevin Johnson, Oficial Taxón, Arca de los Anfibios Estamos muy contentos de anunciar cuatro grandiosos nuevos proyectos que han sido recientemente premiados con una Beca Se- milla del Arca de Anfibios. Este 2016 recibimos solicitudes para becas para trece nuevos programas - más que en cualquier otro año. Esperamos ver un gran progreso y el éxito de todos estos programas. -

NORTHWEST NAZARENE UNIVERSITY Assisting Frog
NORTHWEST NAZARENE UNIVERSITY Assisting Frog Identification in Costa Rica Using a Mobile App THESIS Submitted to the Department of Mathematics and Computer Science in partial fulfillment of the requirements for the degree of BACHELOR OF ARTS Justin Tyler Laplante 2021 THESIS Submitted to the Department of Mathematics and Computer Science in partial fulfillment of the requirements for the degree of BACHELOR OF ARTS Justin Tyler Laplante 2021 Assisting Frog Identification in Costa Rica Using a Mobile App Author: ____________________________________________________________ Justin Tyler Laplante Approved: ____________________________________________________________ Dale Hamilton, Ph.D., Professor, Department of Mathematics and Computer Science, Faculty Advisor Approved: ____________________________________________________________ John Cossel Jr., Ph.D., Professor, Chair, Department of Biology Second Reader Approved: ____________________________________________________________ Barry L. Myers, Ph.D., Chair, Department of Mathematics & Computer Science ABSTRACT Assisting Frog Identification in Costa Rica Using a Mobile App. LAPLANTE, JUSTIN (Department of Mathematics and Computer Science). Quickly identifying a single frog species from over a hundred other possible species can be a challenge for research while in the Costa Rican jungle. Though researchers can use field guides to assist, these still mean you may have look through all currently identified frog species to find the frog being viewed. This project was created to help researchers narrow the list of possible frog species quickly based on Geolocation. Using Xamarin.Forms, an app was developed that worked offline, used an ArcGIS API and was cross platform. However, to ensure performs and accuracy certain design choices were made for designing the ArcGIS map that was used within the app. The used geospatial data for the frog species and generalized it into a hexagonal pattern. -

Witnessing Extinction in Real Time
PERSPECTIVE Witnessing extinction in real time Karen R. Lips* University of Maryland, Department of Biology, College Park, Maryland, United States of America * [email protected] This Perspective is part of the Conservation Stories from the Front Lines Collection I started my amphibian research a little like Thoreau, living alone in a cabin in the woods and recording the seasonal variation in the natural world. I lived in a shack without plumbing or electricity, an hour away from the closest house, in a cloud forest on the top of a mountain that straddled the border between Costa Rica and Panama. I scrambled along mountain streams chasing seasonal reproductive data on treefrogs. The species I studied, Isthmohyla calypsa, a1111111111 remains one of the most spectacular frogs I have ever seen; adults were a brilliant, iridescent a1111111111 green with a bright white throat pouch, and their skin was as textured as the spikey moss they a1111111111 lived on, apparently camouflaging them from predators [1]. a1111111111 I was living a field biologist's dream and expected to spend my career studying tropical a1111111111 montane amphibians in one of the most beautiful and least studied regions in the world. I couldn't know that my study site in this remote cloud forest would give me a front row seat to one of the most distressing ecological mysteries of our time. As a graduate student, I had heard the ominous reports describing the mysterious disap- pearances of amphibian populations around the world. At the time, scientists were debating OPEN ACCESS whether this was a real phenomenon, what might cause it, and whether these events were Citation: Lips KR (2018) Witnessing extinction in connected. -
The UV-Tool, a Guide to the Selection of UV Lighting for Reptiles and Amphibians in Captivity
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/292983157 How much UV-B does my reptile need? The UV-Tool, a guide to the selection of UV lighting for reptiles and amphibians in captivity. Journal of Zoo and Aquarium Research 4(1): 42 - 6... Article · January 2016 CITATIONS READS 2 9,479 8 authors, including: Frances M Baines Joe Chattell UV Guide UK Reaseheath College 8 PUBLICATIONS 83 CITATIONS 1 PUBLICATION 2 CITATIONS SEE PROFILE SEE PROFILE Matt Goetz Durrell Wildlife Conservation Trust 33 PUBLICATIONS 49 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Building a Future for Malagasy Amphibians View project Agile frog (Rana dalmatina) recovery in Jersey View project All content following this page was uploaded by Frances M Baines on 04 February 2016. The user has requested enhancement of the downloaded file. Evidence-based practice How much UV-B does my reptile need? The UV-Tool, a guide to the selection of UV lighting for reptiles and amphibians in captivity Frances Baines1*, Joe Chattell2, James Dale3, Dan Garrick4, Iri Gill5, Matt Goetz6, Tim Skelton7 and Matt Swatman3 1UV Guide UK, Abergavenny, UK 2Reaseheath College, Nantwich, UK 3Chester Zoo, UK 4Marwell Zoo, UK 5Zoological Society of London, UK 6Durrell Wildlife Conservation Trust, Jersey 7Bristol Zoo Gardens, UK JZAR Evidence-based practice Evidence-based JZAR *Correspondence: Frances Baines, UV Guide UK, Greenfield, School Lane, Govilon, Abergavenny NP7 9NT, UK; [email protected] Keywords: Abstract microhabitat design, UV-B, UV index, Guidance is almost non-existent as to suitable levels of UV lighting for reptiles and amphibians, or UV lamps, UV requirements, vivarium how to achieve satisfactory UV gradients using artificial lighting. -

Amphibian Ark Number 43 Keeping Threatened Amphibian Species Afloat June 2018
AArk Newsletter NewsletterNumber 43, June 2018 amphibian ark Number 43 Keeping threatened amphibian species afloat June 2018 In this issue... Reintroduction of the Northern Pool Frog to the UK - Progress Report, April 2018 ............... 2 ® Establishment of a captive breeding program for the Kroombit Tinkerfrog .............................. 4 In situ conservation of the Lemur Leaf Frog through habitat improvement and forest management practices in the Guayacán Rainforest Reserve in Costa Rica .................... 6 Neotropical amphibian biology, management and conservation course .................................. 8 Donation provides for equipment upgrades within the Biogeos Foundation facilities, at the Rescue of Endangered Venezuelan Amphibians program in Venezuela ................... 9 New AArk Conservation Grants program, and call for applications .................................. 10 Amphibian Advocates - José Alfredo Hernández Díaz, Africam Safari, Mexico ........ 11 Amphibian Advocates - Dr. Phil Bishop, Co-Chair IUCN SSC ASG............................... 12 AArk Newsletter - Instructions for authors ...... 13 A private donation helps the Valcheta Frog program in Argentina ...................................... 14 A rich food formula to raise tadpoles in captivity........................................................... 16 Vibicaria Conservation Program: creation of an ex situ model for a rediscovered species in Costa Rica ...................................................... 18 Reproduction of Dendropsophus padreluna at -

Taller Para Revisar La Lista Roja De Anfibios De Costa Rica De La UICN
Taller para Revisar la Lista Roja de Anfibios de Costa Rica de la UICN y Evaluación del Cumplimiento de las Acciones de la Estrategia de Conservación de los Anfibios de Costa Rica 3-4 de agosto, 2010 Escuela de Biología Universidad de Costa Rica San Pedro, San José, Costa Rica Informe Final Organizado por Bolaños, F., G. Chaves, J. E. Rodríguez, B. Young & Y. Matamoros (Eds.) 2010. Taller para Revisar la Lista Roja de Anfibios de Costa Rica de la UICN y Evaluación del Cumplimiento de las Acciones de la Estrategia de Conservación de los Anfibios de Costa Rica. 3-4 de agosto, 2010. Escuela de Biología de la Universidad de Costa Rica, San Pedro, San José, Costa Rica. Conservation Breeding Specialist Group (SSC/IUCN)/CBSG Mesoamerica. Foto portada: Incilius holdridgei. José Andrés Salazar. Una contribución del Grupo de Especialistas en Conservación y Reproducción (CBSG) SSC/UICN. CBSG, SSC y UICN, promueven talleres y otros foros para el análisis y consideración de problemas relativos a la conservación, y considera que los informes de estas reuniones son de gran utilidad cuando son distribuidos extensamente. Las opiniones y recomendaciones expresadas en este informe reflejan los asuntos discutidos y las ideas expresadas por los participantes del taller y no necesariamente refleja la opinión o la posición de CBSG, SSC o UICN. Copias adicionales de esta publicación se pueden ordenar a través de: IUCN/SSC Conservation Breeding Specialist Group (CBSG), 12101 Johnny Cake Ridge Road, Apple Valley, MN 55124. E-mail: [email protected] Website: www.cbsg.org -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -

Lithobates Vibicarius
Conservation of the Critically Endangered Green-eyed Frog Lithobates Vibicarius Project report: by Mark Wainwright and Andrew Gray. TABLE OF CONTENTS 1. Background 2. Preliminary project aims 3. Timeline of activities, aims completed, site visits and preliminary evaluations. September 2007 – December 2008. 4. Preliminary Chytrid results and evaluations 5. Overview and future of the project Background The breeding site of the Critically Endangered frog Lithobates Vibicaria at Chutas, Monteverde Lithobates vibicarius is a critically endangered frog species that was once abundant at several high altitude sites in lower Central America, including the area of Monteverde, Costa Rica. Here, the population crashed in the 1980’s and, until recently, the species was thought to have disappeared completely. Mark Wainwright, a highly respected local Costa Rican naturalist and conservationist, rediscovered the species at Monteverde in 2005. What is thought to be the last remaining viable population for the species is now known from the one breeding pond, named Chutas, at Monteverde. In September 2007, Andrew Gray from Manchester University accompanied Mark Wainwright in the field to jointly assess the breeding site. Following the heaviest downpour of rain all year, a landslide, and a very difficult trekking environment, they travelled to El Valle (the midway camp) and then on to the Chutas site. They were well supported in the field by a guard from the Monteverde Conservation League, Geiner Alvarado, who proved particularly interested in the project and who was a very valuable asset. At this time a very large aggregation of breeding L. vibicarius was witnessed. The pond contained thousands of eggs and tadpoles at various stages of development. -

Unraveling the Skin Microbiome of Endangered Costa Rican Amphibians
fmicb-10-02060 September 10, 2019 Time: 18:4 # 1 ORIGINAL RESEARCH published: 12 September 2019 doi: 10.3389/fmicb.2019.02060 Moving Beyond the Host: Unraveling the Skin Microbiome of Endangered Costa Rican Amphibians Randall R. Jiménez1*, Gilbert Alvarado2,3, Josimar Estrella3 and Simone Sommer1* 1 Institute of Evolutionary Ecology and Conservation Genomics, University of Ulm, Ulm, Germany, 2 Laboratory of Comparative Wildlife Pathology, School of Veterinary Medicine and Animal Sciences, University of São Paulo, São Paulo, Brazil, 3 Laboratory of Experimental and Comparative Pathology (LAPECOM), Biology School, University of Costa Rica, San José, Costa Rica Some neotropical amphibians, including a few species in Costa Rica, were presumed to be “extinct” after dramatic population declines in the late 1980s but have been rediscovered in isolated populations. Such populations seem to have evolved a resistance/tolerance to Batrachochytrium dendrobatidis (Bd), a fungal pathogen that causes a deadly skin disease and is considered one of the main drivers of worldwide amphibian declines. The skin microbiome is an important component of the host’s innate immune system and is associated with Bd-resistance. However, the way that the bacterial diversity of the skin microbiome confers protection against Bd in surviving Edited by: species remains unclear. We studied variation in the skin microbiome and the prevalence Eria Alaide Rebollar, of putatively anti-Bd bacterial taxa in four co-habiting species in the highlands of the National Autonomous University Juan Castro Blanco National Park in Costa Rica using 16S rRNA amplicon sequencing. of Mexico (Morelos), Mexico Lithobates vibicarius, Craugastor escoces, and Isthmohyla rivularis have recently been Reviewed by: Ana V. -

Herpetology at the Isthmus Species Checklist
Herpetology at the Isthmus Species Checklist AMPHIBIANS BUFONIDAE true toads Atelopus zeteki Panamanian Golden Frog Incilius coniferus Green Climbing Toad Incilius signifer Panama Dry Forest Toad Rhaebo haematiticus Truando Toad (Litter Toad) Rhinella alata South American Common Toad Rhinella granulosa Granular Toad Rhinella margaritifera South American Common Toad Rhinella marina Cane Toad CENTROLENIDAE glass frogs Cochranella euknemos Fringe-limbed Glass Frog Cochranella granulosa Grainy Cochran Frog Espadarana prosoblepon Emerald Glass Frog Sachatamia albomaculata Yellow-flecked Glass Frog Sachatamia ilex Ghost Glass Frog Teratohyla pulverata Chiriqui Glass Frog Teratohyla spinosa Spiny Cochran Frog Hyalinobatrachium chirripoi Suretka Glass Frog Hyalinobatrachium colymbiphyllum Plantation Glass Frog Hyalinobatrachium fleischmanni Fleischmann’s Glass Frog Hyalinobatrachium valeroi Reticulated Glass Frog Hyalinobatrachium vireovittatum Starrett’s Glass Frog CRAUGASTORIDAE robber frogs Craugastor bransfordii Bransford’s Robber Frog Craugastor crassidigitus Isla Bonita Robber Frog Craugastor fitzingeri Fitzinger’s Robber Frog Craugastor gollmeri Evergreen Robber Frog Craugastor megacephalus Veragua Robber Frog Craugastor noblei Noble’s Robber Frog Craugastor stejnegerianus Stejneger’s Robber Frog Craugastor tabasarae Tabasara Robber Frog Craugastor talamancae Almirante Robber Frog DENDROBATIDAE poison dart frogs Allobates talamancae Striped (Talamanca) Rocket Frog Colostethus panamensis Panama Rocket Frog Colostethus pratti Pratt’s Rocket -

Shifts in the Diversity of an Amphibian Community from a Premontane Forest of San Ramón, Costa Rica Cambios En La Diversidad De
DOI 10.15517/RBT.V67I2SUPL.37240 Artículo Shifts in the diversity of an amphibian community from a premontane forest of San Ramón, Costa Rica Cambios en la diversidad de una comunidad de anfibios en un bosque premontano de San Ramón, Costa Rica Víctor J. Acosta-Chaves1, 2* Víctor Madrigal-Elizondo3 Gerardo Chaves4 Brayan Morera-Chacón5 Adrián García-Rodríguez 4, 6 Federico Bolaños 4 1 Carrera de Turismo Ecológico, Universidad de Costa Rica Sede Atlántico, Recinto de Paraíso, Cartago, Costa Rica; [email protected]* 2 Red Mesoamericana y del Caribe para la Conservación de Anfibios y Reptiles. 3 Red de Áreas Protegidas, Universidad de Costa Rica, Sede Rodrigo Facio, San Pedro, Costa Rica; [email protected] 4 Escuela de Biología, Universidad de Costa Rica, San Pedro, 11501-2060 San José, Costa Rica; [email protected], [email protected] 5 Instituto Internacional para la Conservación y Manejo de Vida Silvestre, Universidad Nacional, Heredia, Costa Rica; [email protected] 6 Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City, Mexico; [email protected] * Correspondence Received 05-X-2018 Corrected 18-I-2019 Accepted 06-II-2019 Abstract Biological communities are experiencing rapid shifts of composition in Neotropical ecosystems due to several factors causing population declines. However, emerging evidence has provided insights on the adaptive potential of multiple species to respond to illnesses and environmental pressures. In Costa Rica, the decline of amphibian populations is a remarkable example of these changes. Here we provide evidence of variation in the amphibian richness of a premontane forest of San Ramón (Costa Rica) across a ~30 year period. -
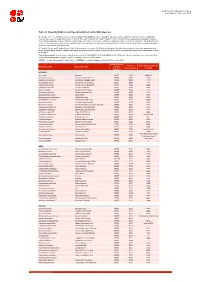
Table 9 Last Updated: 14 November 2018
IUCN Red List version 2018-2: Table 9 Last Updated: 14 November 2018 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". Year of Assessment - year the species was first assessed as 'Possibly Extinct' or 'Possibly Extinct in the Wild'; some species may have been reassessed since then but have retained their 'Possibly Extinct' or 'Possibly Extinct in the Wild' status. CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red List Year of Date last recorded