Witnessing Extinction in Real Time
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NORTHWEST NAZARENE UNIVERSITY Assisting Frog
NORTHWEST NAZARENE UNIVERSITY Assisting Frog Identification in Costa Rica Using a Mobile App THESIS Submitted to the Department of Mathematics and Computer Science in partial fulfillment of the requirements for the degree of BACHELOR OF ARTS Justin Tyler Laplante 2021 THESIS Submitted to the Department of Mathematics and Computer Science in partial fulfillment of the requirements for the degree of BACHELOR OF ARTS Justin Tyler Laplante 2021 Assisting Frog Identification in Costa Rica Using a Mobile App Author: ____________________________________________________________ Justin Tyler Laplante Approved: ____________________________________________________________ Dale Hamilton, Ph.D., Professor, Department of Mathematics and Computer Science, Faculty Advisor Approved: ____________________________________________________________ John Cossel Jr., Ph.D., Professor, Chair, Department of Biology Second Reader Approved: ____________________________________________________________ Barry L. Myers, Ph.D., Chair, Department of Mathematics & Computer Science ABSTRACT Assisting Frog Identification in Costa Rica Using a Mobile App. LAPLANTE, JUSTIN (Department of Mathematics and Computer Science). Quickly identifying a single frog species from over a hundred other possible species can be a challenge for research while in the Costa Rican jungle. Though researchers can use field guides to assist, these still mean you may have look through all currently identified frog species to find the frog being viewed. This project was created to help researchers narrow the list of possible frog species quickly based on Geolocation. Using Xamarin.Forms, an app was developed that worked offline, used an ArcGIS API and was cross platform. However, to ensure performs and accuracy certain design choices were made for designing the ArcGIS map that was used within the app. The used geospatial data for the frog species and generalized it into a hexagonal pattern. -

Taxonomic Checklist of Amphibian Species Listed in the CITES
CoP17 Doc. 81.1 Annex 5 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and TheAmericanMuseum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. CoP17 Doc. 81.1 Annex 5 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation EC 338/97 Regulation only 338/97 ANURA Aromobatidae Allobates femoralis X Aromobatidae Allobates hodli X Aromobatidae Allobates myersi X Aromobatidae Allobates zaparo X Aromobatidae Anomaloglossus rufulus X Bufonidae Altiphrynoides malcolmi X Bufonidae Altiphrynoides osgoodi X Bufonidae Amietophrynus channingi X Bufonidae Amietophrynus superciliaris X Bufonidae Atelopus zeteki X Bufonidae Incilius periglenes X Bufonidae Nectophrynoides asperginis X Bufonidae Nectophrynoides cryptus X Bufonidae Nectophrynoides frontierei X Bufonidae Nectophrynoides laevis X Bufonidae Nectophrynoides laticeps X Bufonidae Nectophrynoides minutus X Bufonidae Nectophrynoides paulae X Bufonidae Nectophrynoides poyntoni X Bufonidae Nectophrynoides pseudotornieri X Bufonidae Nectophrynoides tornieri X Bufonidae Nectophrynoides vestergaardi -
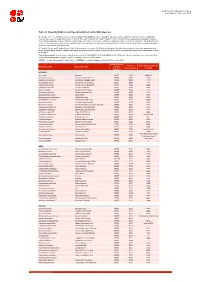
Table 9 Last Updated: 14 November 2018
IUCN Red List version 2018-2: Table 9 Last Updated: 14 November 2018 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". Year of Assessment - year the species was first assessed as 'Possibly Extinct' or 'Possibly Extinct in the Wild'; some species may have been reassessed since then but have retained their 'Possibly Extinct' or 'Possibly Extinct in the Wild' status. CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red List Year of Date last recorded -

Development of an Edna Assay for Cane Toad (Rhinella Marina)
Development of an eDNA assay for cane toad (Rhinella marina) Report by Richard C. Edmunds and Damien Burrows © James Cook University, 2019 Development of an eDNA assay for cane toad (Rhinella marina) is licensed by James Cook University for use under a Creative Commons Attribution 4.0 Australia licence. For licence conditions see creativecommons.org/licenses/by/4.0 This report should be cited as: Edmunds, R.C. and Burrows, D. 2019. Development of eDNA assay for cane toad (Rhinella marina). Report 19/08, Centre for Tropical Water and Aquatic Ecosystem Research (TropWATER), James Cook University, Townsville. Cover photographs Front cover: Cane toad Rhinella marina (photo: Peter Yeeles/Shutterstock.com). Back cover: Cane toad Rhinella marina (photo: Cecilia Villacorta-Rath). This report is available for download from the Northern Australia Environmental Resources (NAER) Hub website at nespnorthern.edu.au The Hub is supported through funding from the Australian Government’s National Environmental Science Program (NESP). The NESP NAER Hub is hosted by Charles Darwin University. ISBN 978-1-925800-29-6 June, 2019 Printed by Uniprint Contents Acronyms....................................................................................................................................iv Abbreviations .............................................................................................................................. v Acknowledgements ....................................................................................................................vi -

Resurrección De Especies Extintas: El Por Qué Sí Y El Por Qué No De La Des-Extinción En Lenguaje Sencillo
Resurrección de especies extintas: el por qué sí y el por qué no de la des-extinción en lenguaje sencillo Julián Monge-Nájera* Recibido: 29-09-2015 Aceptado: 10-10-2015 RESUMEN Recientemente se ha vuelto urgente comprender qué es, cómo se hace y que aspectos positivos y negativos tiene la des-extinción de especies. En este artículo se resume, en lenguaje sencillo, los avances más recientes en este campo con base en la literatura científica. El primer proyecto para resucitar especies extintas se inició en Alema- nia en 1920 con el tarpán (Equus ferus ferus), y el uro (Bos primigenius) usando la técnica de entrecruzamiento desarrollada por los hermanos Heck. Más recientemente se han propuesto otras técnicas, como el uso de semen u óvulos congelados de restos árticos, la clonación de tejidos de museos y la manipulación de ADN con ingeniería genética. Algunas especies costarricenses que se extinguieron pero teóricamente podrían resucitarse son el sapo dorado de Monteverde (Incilius periglenes), el sapo de Chirripó (Atelopus chirripoensis), el sapo del Pico Blanco (Incilius fastidiosus), el caracol escazuceño (Velifera gabbi), el tigre dientes de sable (Smilodon fatalis), el mamut (Mammuthus columbi), el gonfoterio, (Cuvieronius hyodon), el perezoso gigante (Eremotherium laurillardi) y el armadillo gigante (Holmesina septentrionalis), pues se tiene ADN de varias de esas especies. A favor de la des-ex- tinción se han dado las razones de corregir nuestros propios delitos ambientales, obtener alimentos y medicinas y financiar esfuerzos de conservación. Los argumentos en contra tienen que ver con el peligro de enfermedades, desvío de fondos, catástrofes ecológicas y abusos tecnológicos. -

Cerrophidion Wilsoni Jadin, Townsend, Castoe, and Campbell, 2012. The
Cerrophidion wilsoni Jadin, Townsend, Castoe, and Campbell, 2012. The Honduran Montane Pitviper is a priority one species with an EVS of 15, placing it in the high vulnerability category (see this paper). This pitviper is distributed primarily in lower montane rainforest at elevations from 1,400 to 3,491 m, but can occur peripherally in premontane rainforest and pine-oak forest as low as 1,220 m (Jadin et al. 2012). As indicated by Jadin et al. (2012: 10), this snake “occurs in at least 13 isolated highland forest areas across Eastern Nuclear Central America…and all known populations…are found within the borders of Honduras and El Salvador.” This juvenile individual was found in Refugio de Vida Silvestre Texíguat, in north-central Honduras. One of the describers of this taxon is the dedicatee of this paper, and the snake was named in honor of one of the authors. Photo by Josiah H. Townsend. Amphib. Reptile Conserv. 1 January 2019 | Volume 13 | Number 1 | e168 DEDICATION We are happy to dedicate this paper to our friend and Josiah H. Townsend. 2018. An integrative assessment colleague, Josiah H. Townsend, Associate Professor of of the taxonomic status of putative hybrid leopard frogs Biology at Indiana University of Pennsylvania, in Indiana, (Anura: Ranidae) from the Chortís Highlands of Central Pennsylvania. Over the last two decades, since he was America, with description of a new species. Systematics a student in one of Larry Wilson’s classes, Joe has built and Biodiversity 2018: 1–17. This paper is an example an imposing reputation as the principal authority on the of the seminal work being conducted by Joe Townsend herpetofauna of the biogeographically significant Chortís and his colleagues, which is exposing the underestimated Highlands of northern Central America. -
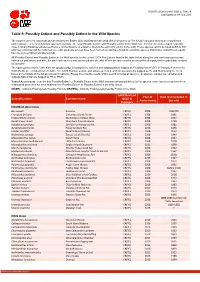
Table 9 Last Updated: 09 July 2020
IUCN Red List version 2020-2: Table 9 Last Updated: 09 July 2020 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". The figures presented in Table 9 are for species only; all subspecies, varieties and subpopulations flagged as 'Possibly Extinct' (PE) or 'Possibly Extinct in the Wild' (PEW) are excluded from the table. The IUCN Red List website also allows users to search for assessments flagged as PE and PEW (using the check boxes at the bottom of the Advanced search options). Please note that the results of that search includes all taxa (i.e., all species, subspecies, varieities and subpopulations that are flagged as PE or PEW). -

Table 9 Last Updated: 30 June 2016
IUCN Red List version 2016-1: Table 9 Last Updated: 30 June 2016 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". Year of Assessment - year the species was first assessed as 'Possibly Extinct' or 'Possibly Extinct in the Wild'; some species may have been reassessed since then but have retained their 'Possibly Extinct' or 'Possibly Extinct in the Wild' status. CR(PE) - Critically Endangered (Possibly Extinct), CR(PEW) - Critically Endangered (Possibly Extinct in the Wild), IUCN Red Year of Date last recorded Scientific -
Table 9: Possibly Extinct and Possibly Extinct in the Wild Species
IUCN Red List version 2020-1: Table 9 Last Updated: 19 March 2020 Table 9: Possibly Extinct and Possibly Extinct in the Wild Species The number of recent extinctions documented by the Extinct (EX) and Extinct in the Wild (EW) categories on The IUCN Red List is likely to be a significant underestimate, even for well-known taxa such as birds. The tags 'Possibly Extinct' and 'Possibly Extinct in the Wild' have therefore been developed to identify those Critically Endangered species that are, on the balance of evidence, likely to be extinct (or extinct in the wild). These species cannot be listed as EX or EW until their extinction can be confirmed (i.e., until adequate surveys have been carried out and have failed to record the species and local or unconfirmed reports have been investigated and discounted). All 'Possibly Extinct' and 'Possibly Extinct in the Wild' species on the current IUCN Red List are listed in the table below, along the year each assessment was carried out and, where available, the date each species was last recorded in the wild. Where the last record is an unconfirmed report, last recorded date is noted as "possibly". The figures presented in Table 9 are for species only; all subspecies, varieties and subpopulations flagged as 'Possibly Extinct' (PE) or 'Possibly Extinct in the Wild' (PEW) are excluded from the table. The IUCN Red List website also allows users to search for assessments flagged as PE and PEW (using the check boxes at the bottom of the Advanced search options). Please note that the results of that search includes all taxa (i.e., all species, subspecies, varieities and subpopulations that are flagged as PE or PEW). -

Related Extinctions of Panamanian Amphibians Through Captive Breeding Programs B
Animal Conservation. Print ISSN 1367-9430 Evaluating the probability of avoiding disease-related extinctions of Panamanian amphibians through captive breeding programs B. Gratwicke1, H. Ross2, A. Batista3, G. Chaves4, A. J. Crawford5,6,7, L. Elizondo8, A. Estrada9, M. Evans10, D. Garelle11, J. Guerrel12, A. Hertz3,13, M. Hughey9, C. A. Jaramillo6,7,14,15, B. Klocke16, M. Mandica17, D. Medina9, C. L. Richards-Zawacki6,18, M. J. Ryan19, A. Sosa-Bartuano20, J. Voyles21, B. Walker15, D. C. Woodhams6,22 &R.Ibanez~ 7,12,23 1 Center for Species Survival, Smithsonian Conservation Biology Institute, National Zoological Park, Washington, DC, USA 2 Panama Amphibian Rescue and Conservation Project, El Valle Amphibian Conservation Center, Smithsonian Tropical Research Institute, Panama, Republic of Panama 3 Senckenberg Forschungsinstitut und Naturmuseum Frankfurt, Frankfurt, Germany 4 Escuela de Biologıa, Universidad de Costa Rica, San Jose, Costa Rica 5 Department of Biological Sciences, Universidad de los Andes, Bogota, Colombia 6 Smithsonian Tropical Research Institute, Panama, Republic of Panama 7Cırculo Herpetologico de Panama, Panama, Republic of Panama 8 Programa de Maestrıa en Ciencias Biologicas, Universidad de Panama, Panama, Republic of Panama 9 Department of Biological Sciences, Virginia Tech, Blacksburg, VA, USA 10 Reptile Discovery Center, Smithsonian’s National Zoological Park, Washington, DC, USA 11 Cheyenne Mountain Zoo, Colorado Springs, CO, USA 12 Panama Amphibian Rescue and Conservation Project, Smithsonian Tropical Research Institute, -

AC28 Doc. 21.1 Annex 7 (English Only / Únicamente En Inglés / Seulement En Anglais)
AC28 Doc. 21.1 Annex 7 (English only / Únicamente en inglés / Seulement en anglais) Taxonomic Checklist of Amphibian Species listed in the CITES Appendices and the Annexes of EC Regulation 338/97 Species information extracted from FROST, D. R. (2015) "Amphibian Species of the World, an online Reference" V. 6.0 (as of May 2015) Copyright © 1998-2015, Darrel Frost and The American Museum of Natural History. All Rights Reserved. Additional comments included by the Nomenclature Specialist of the CITES Animals Committee (indicated by "NC comment") Reproduction for commercial purposes prohibited. AC28 Doc. 21.1 Annex 7 - p. 1 Amphibian Species covered by this Checklist listed by listed by CITES EC- as well as Family Species Regulation Page EC 338/97 Regulation only 338/97 ANURA 7 7 Aromobatidae Allobates femoralis X 7 Aromobatidae Allobates hodli X 7 Aromobatidae Allobates myersi X 8 Aromobatidae Allobates zaparo X 8 Aromobatidae Anomaloglossus rufulus X 8 Bufonidae Altiphrynoides malcolmi X 9 Bufonidae Altiphrynoides osgoodi X 9 Bufonidae Amietophrynus channingi X 10 Bufonidae Amietophrynus superciliaris X 10 Bufonidae Atelopus zeteki X 11 Bufonidae Incilius periglenes X 11 Bufonidae Nectophrynoides asperginis X 12 Bufonidae Nectophrynoides cryptus X 12 Bufonidae Nectophrynoides frontierei X 12 Bufonidae Nectophrynoides laevis X 13 Bufonidae Nectophrynoides laticeps X 13 Bufonidae Nectophrynoides minutus X 13 Bufonidae Nectophrynoides paulae X 13 Bufonidae Nectophrynoides poyntoni X 14 Bufonidae Nectophrynoides pseudotornieri X 14 Bufonidae -

Batrachochytrium Dendrobatidis Global Invasive Species Database
FULL ACCOUNT FOR: Batrachochytrium dendrobatidis Batrachochytrium dendrobatidis System: Undefined Kingdom Phylum Class Order Family Fungi Chytridiomycota Chytridiomycetes Chytridiales Common name chytrid frog fungi (English), Chytrid-Pilz (German), chytridiomycosis (English), frog chytrid fungus (English) Synonym Similar species Summary Batrachochytrium dendrobatidis is a non-hyphal parasitic chytrid fungus that has been associated with population declines in endemic amphibian species in upland montane rain forests in Australia and Panama. It causes cutaneous mycosis (fungal infection of the skin), or more specifically chytridiomycosis, in wild and captive amphibians. First described in 1998, the fungus is the only chytrid known to parasitise vertebrates. B. dendrobatidis can remain viable in the environment (especially aquatic environments) for weeks on its own, and may persist in latent infections. view this species on IUCN Red List Global Invasive Species Database (GISD) 2021. Species profile Batrachochytrium Pag. 1 dendrobatidis. Available from: http://www.iucngisd.org/gisd/species.php?sc=123 [Accessed 06 October 2021] FULL ACCOUNT FOR: Batrachochytrium dendrobatidis Species Description Fungal Morphology: Batrachochytrium dendrobatidis is a zoosporic chytrid fungus that causes chytridiomycosis (a fungal infection of the skin) in amphibians and grows solely within keratinised cells. Diagnosis is by identification of characteristic intracellular flask-shaped sporangia (spore containing bodies) and septate thalli. The fungus grows in the superficial keratinised layers of the epidermis (known as the stratum corneum and stratum granulosum). The normal thickness of the stratum corneum is between 2µm to 5µm, but a heavy infection by the chytrid parasite may cause it to thicken to up to 60 µm. The fungus also infects the mouthparts of tadpoles (which are keratinised) but does not infect the epidermis of tadpoles (which lacks keratin).