Nursery Functions of Us West Coast Estuaries: the State of Knowledge For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of Selected California Fisheries for 2013
FISHERIES REVIEW CalCOFI Rep., Vol. 55, 2014 REVIEW OF SELECTED CALIFORNIA FISHERIES FOR 2013: COASTAL PELAGIC FINFISH, MARKET SQUID, GROUNDFISH, HIGHLY MIGRATORY SPECIES, DUNGENESS CRAB, BASSES, SURFPERCH, ABALONE, KELP AND EDIBLE ALGAE, AND MARINE AQUACULTURE CALIFORNIA DEPARTMENT OF FISH AND WILDLIFE Marine Region 4665 Lampson Ave. Suite C Los Alamitos, CA 90720 [email protected] SUMMARY ings of northern anchovy were 6,005 t with an ex-vessel In 2013, commercial fisheries landed an estimated revenue of greater than $1.0 million. When compared 165,072 metric tons (t) of fish and invertebrates from to landings in 2012, this represents a 141% and 191% California ocean waters (fig. 1). This represents an increase in volume and value, respectively. Nearly all increase of almost 2% from the 162,290 t landed in 2012, (93.6%; 5,621.5 t) of California’s 2013 northern anchovy but still an 11% decrease from the 184,825 t landed catch was landed in the Monterey port area. Landings of in 2011, and a 35% decline from the peak landings of jack mackerel remained relatively low with 892 t landed; 252,568 t observed in 2000. The preliminary ex-vessel however, this represents a 515% increase over 2012 land- economic value of commercial landings in 2013 was ings of 145 t. $254.7 million, increasing once again from the $236 mil- Dungeness crab ranked as California’s second largest lion generated in 2012 (8%), and the $198 million in volume fishery with 14,066 t landed, an increase from 2011 (29%). 11,696 t landed in 2012, and it continued to dominate as Coastal pelagic species (CPS) made up four of the the highest valued fishery in the state with an ex-vessel top five volume fisheries in 2013. -

Classification of Wetlands and Deepwater Habitats of the United States
Pfego-/6^7fV SDMS DocID 463450 ^7'7/ Biological Services Program \ ^ FWS/OBS-79/31 DECEMBER 1979 Superfund Records Center ClassificaHioFF^^^ V\Aetlands and Deepwater Habitats of the United States KPHODtKtD BY NATIONAL TECHNICAL INFOR/V^ATION SERVICE U.S. IKPARTMEN TOF COMMERCt SPRINGMflO, VA. 22161 Fish and Wildlife Service U.S. Department of the Interior (USDI) C # The Biological Services Program was established within the U.S. Fish . and Wildlife Service to supply scientific information and methodologies on key environmental issues which have an impact on fish and wildlife resources and their supporting ecosystems. The mission of the Program is as follows: 1. To strengthen the Fish and Wildlife Service in its role as a primary source of Information on natural fish and wildlife resources, par ticularly with respect to environmental impact assessment. 2. To gather, analyze, and present information that will aid decision makers in the identification and resolution of problems asso ciated with major land and water use changes. 3. To provide better ecological information and evaluation for Department of the Interior development programs, such as those relating to energy development. Information developed by the Biological Services Program is intended for use in the planning and decisionmaking process, to prevent or minimize the impact of development on fish and wildlife. Biological Services research activities and technical assistance services are based on an analysis of the issues, the decisionmakers involved and their information neeids, and an evaluation of the state^f-the-art to Identify information gaps and determine priorities. This Is a strategy to assure that the products produced and disseminated will be timely and useful. -

Fish Larvae, Development, Allometric Growth, and the Aquatic Environment
II. Developmental and environmental interactions ICES mar. Sei. Symp., 201: 21-34. 1995 Fish larvae, development, allometric growth, and the aquatic environment J. W. M. Osse and J. G. M. van den Boogaart Osse, J. W. M., and van den Boogaart, J. G. M. 1995. Fish larvae, development, allometric growth, and the aquatic environment. - ICES mar. Sei. Symp., 201: 21-34. During metamorphosis, fish larvae undergo rapid changes in external appearance and dimensions of body parts. The juveniles closely resemble the adult form. The focus of the present paper is the comparison of stages of development of fishes belonging within different taxa in a search for general patterns. The hypothesis that these patterns reflect the priorities of vital functions, e.g., feeding, locomotion, and respiration, is tested taking into account the size-dependent influences of the aquatic environment on the larvae. A review of some literature on ailometries of fish larvae is given and related to the rapidly changing balance between inertial and viscous forces in relation to body length and swimming velocity. New data on the swimming of larval and juvenile carp are presented. It is concluded that the patterns of development and growth shows in many cases a close parallel with the successive functional requirements. J. W. M. Osse and J. G. M. van den Boogaart: Wageningen Agricultural University, Department of Experimental Animal Morphology and Cell Biology, Marijkeweg 40, 6709 PG Wageningen, The Netherlands [tel: (+31) (0)317 483509, fax: (+31) (0)317 483962], Introduction Fish larvae, being so small, must have problems using their limited resources for maintenance, activity, obtain Fish larvae are the smallest free-living vertebrates. -

Grade 3 Unit 2 Overview Open Ocean Habitats Introduction
G3 U2 OVR GRADE 3 UNIT 2 OVERVIEW Open Ocean Habitats Introduction The open ocean has always played a vital role in the culture, subsistence, and economic well-being of Hawai‘i’s inhabitants. The Hawaiian Islands lie in the Pacifi c Ocean, a body of water covering more than one-third of the Earth’s surface. In the following four lessons, students learn about open ocean habitats, from the ocean’s lighter surface to the darker bottom fl oor thousands of feet below the surface. Although organisms are scarce in the deep sea, there is a large diversity of organisms in addition to bottom fi sh such as polycheate worms, crustaceans, and bivalve mollusks. They come to realize that few things in the open ocean have adapted to cope with the increased pressure from the weight of the water column at that depth, in complete darkness and frigid temperatures. Students fi nd out, through instruction, presentations, and website research, that the vast open ocean is divided into zones. The pelagic zone consists of the open ocean habitat that begins at the edge of the continental shelf and extends from the surface to the ocean bottom. This zone is further sub-divided into the photic (sunlight) and disphotic (twilight) zones where most ocean organisms live. Below these two sub-zones is the aphotic (darkness) zone. In this unit, students learn about each of the ocean zones, and identify and note animals living in each zone. They also research and keep records of the evolutionary physical features and functions that animals they study have acquired to survive in harsh open ocean habitats. -

Northern California Coast Northern Focus Area
14.1 Description of Area 14.1.1 The Land The Northern California Coast - Northern Focus Area is composed of coastal Del Norte and Humboldt counties. The boundary extends eastward from the Pacific coast to the top of the first inland mountain range, and encompasses many of the region's existing and former wetlands. The focus area also includes a few important riparian and floodplain areas adjacent to major coastally draining rivers (Figure 13). In this northernmost California County, the coastline tends to be composed of rocky cliffs and high bluffs which rise steeply into the coastal mountain ranges with their deeply cut 14.0 canyons. Two major rivers drain the interior mountain ranges and empty into the Pacific Ocean within the boundary of Del Norte County: the Smith River, which has its origins in north- eastern Del Norte County and southern Oregon, and the Klamath River with headwaters much farther to the NORTHERN north and east in south central Oregon. Humboldt County, to the south, includes portions of CALIFORNIA the California Coast Range and the southern Klamath Mountains. The most extensive coastal wetlands are associated with floodplains in the lower Eel River COAST─ Valley and the Humboldt Bay area. Other significant wetland habitats include Mad River Estuary, Little River Valley, Redwood Creek Estuary, Big Lagoon, NORTHERN Stone Lagoon, and Freshwater Lagoon. Major rivers and streams draining the mountain ranges of Humboldt County include the Eel River, Van Duzen FOCUS AREA River, Mad River, Trinity River, Klamath River, Mattole River, Bear River, and Redwood Creek. Like the Klamath River, the Trinity and Eel rivers have large drainage basins within the Coast Range and the Klamath Mountains. -

Snakeheadsnepal Pakistan − (Pisces,India Channidae) PACIFIC OCEAN a Biologicalmyanmar Synopsis Vietnam
Mongolia North Korea Afghan- China South Japan istan Korea Iran SnakeheadsNepal Pakistan − (Pisces,India Channidae) PACIFIC OCEAN A BiologicalMyanmar Synopsis Vietnam and Risk Assessment Philippines Thailand Malaysia INDIAN OCEAN Indonesia Indonesia U.S. Department of the Interior U.S. Geological Survey Circular 1251 SNAKEHEADS (Pisces, Channidae)— A Biological Synopsis and Risk Assessment By Walter R. Courtenay, Jr., and James D. Williams U.S. Geological Survey Circular 1251 U.S. DEPARTMENT OF THE INTERIOR GALE A. NORTON, Secretary U.S. GEOLOGICAL SURVEY CHARLES G. GROAT, Director Use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Geological Survey. Copyrighted material reprinted with permission. 2004 For additional information write to: Walter R. Courtenay, Jr. Florida Integrated Science Center U.S. Geological Survey 7920 N.W. 71st Street Gainesville, Florida 32653 For additional copies please contact: U.S. Geological Survey Branch of Information Services Box 25286 Denver, Colorado 80225-0286 Telephone: 1-888-ASK-USGS World Wide Web: http://www.usgs.gov Library of Congress Cataloging-in-Publication Data Walter R. Courtenay, Jr., and James D. Williams Snakeheads (Pisces, Channidae)—A Biological Synopsis and Risk Assessment / by Walter R. Courtenay, Jr., and James D. Williams p. cm. — (U.S. Geological Survey circular ; 1251) Includes bibliographical references. ISBN.0-607-93720 (alk. paper) 1. Snakeheads — Pisces, Channidae— Invasive Species 2. Biological Synopsis and Risk Assessment. Title. II. Series. QL653.N8D64 2004 597.8’09768’89—dc22 CONTENTS Abstract . 1 Introduction . 2 Literature Review and Background Information . 4 Taxonomy and Synonymy . -

Browning Washington 0250O 1
Evidence of habitat associations and distribution patterns of rockfish in Puget Sound from archival data (1974-1977) Hilary F. Browning A thesis submitted in partial fulfillment of the requirements for the degree of Master of Marine Affairs University of Washington 2013 Committee: Terrie Klinger Ben Stewart-Koster Program Authorized to Offer Degree: School of Marine and Environmental Affairs 1 University of Washington Graduate School This is to certify that I have examined this copy of a master’s thesis by Hilary F. Browning and have found that it is complete and satisfactory in all respects, and that any and all revisions required by the final examining committee have been made. Committee Members: _____________________________________________________ Terrie Klinger _____________________________________________________ Ben Stewart-Koster Date: __________________________________ 2 In presenting this thesis in partial fulfillment of the requirements for a master’s degree at the University of Washington, I agree that the Library shall make its copies freely available for inspection. I further agree that extensive copying of this thesis is allowable only for scholarly purposes, consistent with “fair use” as prescribed in the U.S. Copyright Law. Any other reproduction for any purposes or by any means shall not be allowed without my written permission. Signature: _________________________________ Date: _____________________________________ 3 University of Washington ABSTRACT Evidence of habitat associations and distribution patterns of rockfish in Puget Sound from archival data (1974-1977) Hilary F. Browning Chair of Supervisory Committee: Associate Professor Terrie Klinger School of Marine and Environmental Affairs Rockfish (Sebastes spp.) populations have declined dramatically and failed to recover in Puget Sound, WA following a period of heavy exploitation in the 1970s and 1980s. -

2 3 Food Web Data Report Final 3June2008
RMP Food Web Analysis; Data Report on Gut Contents of Four Fish Species Andrew Jahn 5 March 2008 1 Author contact information: Andrew Jahn [email protected] Cover images photographed by the author from gut content samples. Top left: Nippoleucon hinumensis (Asian cumacean). Top right: Synidotea sp. (Crustacea: Isopoda). Bottom left and right: Spinileberis sp. (Crustacea: Ostracoda). 2 EXECUTIVE SUMMARY Diet data (as average percentage by volume of contents) from moderate-sized samples (30 – 45) of four fish species (shiner perch, white croaker, topsmelt, and Mississippi silverside) were obtained from RMP and other available fish samples. In this study as well as in other information available for San Francisco Bay, all four species fed mainly on benthic crustaceans, with minor reliance on water-column prey. White croaker fed on larger organisms than the other three species, in apparent agreement with its usual placement at a higher trophic level in bioaccumulation models. Topsmelt and Mississippi silverside were most similar, such that the available diet information on these two species does not offer a ready explanation for their marked difference in tissue mercury content. Apparent spatial variation in the diet of all four species is confounded with differences in time of sampling and/or size of fish. Continued work on fish diets, along with direct measurement of contaminant levels in key prey and associated sediment, are promising approaches to understanding the linkage between sediment contamination and human and wildlife receptors. INTRODUCTION This data report is a contribution to the SFEI special study entitled "Development of a refined conceptual model for aquatic food webs in San Francisco Bay." The study proposed to address the following fundamental questions: 1. -

Comparison of an Active and a Passive Age-0 Fish Sampling Gear in a Tropical Reservoir
Comparison of an Active and a Passive Age-0 Fish Sampling Gear in a Tropical Reservoir M. Clint Lloyd, Department of Wildlife, Fisheries and Aquaculture, Mississippi State University, Box 9690 Mississippi State, MS 39762 J. Wesley Neal, Department of Wildlife, Fisheries and Aquaculture, Mississippi State University, Box 9690 Mississippi State, MS 39762 Abstract: Age-0 fish sampling is an important tool for predicting recruitment success and year-class strength of cohorts in fish populations. In Puerto Rico, limited research has been conducted on age-0 fish sampling with no studies addressing reservoir systems. In this study, we compared the efficacy of passively-fished light traps and actively-fished push nets for sampling the limnetic age-0 fish community in a tropical reservoir. Diversity of catch between push nets and light traps were similar, although species composition of catches differed between gears (pseudo-F = 32.21, df =1,23, P < 0.001) and among seasons (pseudo-F = 4.29, df = 3,23, P < 0.006). Push-net catches were dominated by threadfin shad (Dorosoma petenense), comprising 94.2% of total catch. Conversely, light traps collected primarily channel catfish Ictalurus( punctatus; 76.8%), with threadfin shad comprising only 13.8% of the sample. Light-trap catches had less species diversity and evenness compared to push nets, consequently their efficiency may be limited to presence/ absence of species. These two gears sampled different components of the age-0 fish community and therefore, gear selection should be based on- re search goals, with push nets an ideal gear for threadfin shad age-0 fish sampling, and light traps more appropriate for community sampling. -
Suisun Marsh Fish Report 2015 Final
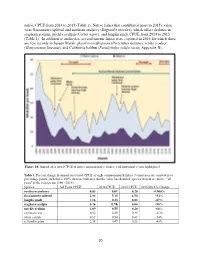
native CPUE from 2014 to 2015 (Table 1). Native fishes that contributed most to 2015's value were Sacramento splittail and northern anchovy (Engraulis mordax), which offset declines in staghorn sculpin, prickly sculpin (Cottus asper), and longfin smelt CPUE from 2014 to 2015 (Table 1). In addition to anchovies, several marine fishes were captured in 2016 for which there are few records in Suisun Marsh: plainfin midshipman (Porichthys notatus), white croaker (Genyonemus lineatus), and California halibut (Paralichthys californicus; Appendix B). Figure 14. Annual otter trawl CPUE of native and non-native fishes, with important events highlighted. Table 1. Percent change in annual otter trawl CPUE of eight common marsh fishes (% increases are equivalent to percentage points, such that a 100% increase indicates that the value has doubled; species in bold are native; "all years" is the average for 1980 - 2015). Species All Years CPUE 2014 CPUE 2015 CPUE 2015/2014 % Change northern anchovy 0.03 0.01 0.20 +1900% Sacramento splittail 2.84 5.15 6.90 +34% longfin smelt 1.16 0.23 0.03 -87% staghorn sculpin 0.26 0.16 0.00 -98% prickly sculpin 1.09 0.55 0.20 -64% common carp 0.52 0.49 0.19 -61% white catfish 0.63 0.94 0.43 -54% yellowfin goby 2.31 0.47 0.26 -45% 20 Beach Seines Annual beach seine CPUE in 2015 was similar to the average from 1980 to 2015 (57 fish per seine; Figure 15), declining mildly from 2014 to 2015 (62 and 52 per seine, respectively). CPUE declined slightly for both non-native and native fishes from 2014 to 2015 (Figure 15); as usual, non-native fish, dominated by Mississippi silversides (Menidia audens), were far more abundant in seine hauls than native fish (Table 2). -

Yellowfin Trawling Fish Images 2013 09 16
Fishes captured aboard the RV Yellowfin in otter trawls: September 2013 Order: Aulopiformes Family: Synodontidae Species: Synodus lucioceps common name: California lizardfish Order: Gadiformes Family: Merlucciidae Species: Merluccius productus common name: Pacific hake Order: Ophidiiformes Family: Ophidiidae Species: Chilara taylori common name: spotted cusk-eel plainfin specklefin Order: Batrachoidiformes Family: Batrachoididae Species: Porichthys notatus & P. myriaster common name: plainfin & specklefin midshipman plainfin specklefin Order: Batrachoidiformes Family: Batrachoididae Species: Porichthys notatus & P. myriaster common name: plainfin & specklefin midshipman plainfin specklefin Order: Batrachoidiformes Family: Batrachoididae Species: Porichthys notatus & P. myriaster common name: plainfin & specklefin midshipman Order: Gasterosteiformes Family: Syngnathidae Species: Syngnathus leptorynchus common name: bay pipefish Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes semicinctus common name: halfbanded rockfish Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes dalli common name: calico rockfish Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes saxicola common name: stripetail rockfish Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes diploproa common name: splitnose rockfish Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes rosenblatti common name: greenblotched rockfish juvenile Order: Scorpaeniformes Family: Scorpaenidae Species: Sebastes levis common name: cowcod Order: -

Crangon Franciscorum Class: Multicrustacea, Malacostraca, Eumalacostraca
Phylum: Arthropoda, Crustacea Crangon franciscorum Class: Multicrustacea, Malacostraca, Eumalacostraca Order: Eucarida, Decapoda, Pleocyemata, Caridea Common gray shrimp Family: Crangonoidea, Crangonidae Taxonomy: Schmitt (1921) described many duncle segment (Wicksten 2011). Inner fla- shrimp in the genus Crago (e.g. Crago fran- gellum of the first antenna is greater than ciscorum) and reserved the genus Crangon twice as long as the outer flagellum (Kuris et for the snapping shrimp (now in the genus al. 2007) (Fig. 2). Alpheus). In 1955–56, the International Mouthparts: The mouth of decapod Commission on Zoological Nomenclature crustaceans comprises six pairs of appendag- formally reserved the genus Crangon for the es including one pair of mandibles (on either sand shrimps only. Recent taxonomic de- side of the mouth), two pairs of maxillae and bate revolves around potential subgeneric three pairs of maxillipeds. The maxillae and designation for C. franciscorum (C. Neocran- maxillipeds attach posterior to the mouth and gon franciscorum, C. franciscorum francis- extend to cover the mandibles (Ruppert et al. corum) (Christoffersen 1988; Kuris and Carl- 2004). Third maxilliped setose and with exo- ton 1977; Butler 1980; Wicksten 2011). pod in C. franciscorum and C. alaskensis (Wicksten 2011). Description Carapace: Thin and smooth, with a Size: Average body length is 49 mm for single medial spine (compare to Lissocrangon males and 68 mm for females (Wicksten with no gastric spines). Also lateral (Schmitt 2011). 1921) (Fig. 1), hepatic, branchiostegal and Color: White, mottled with small black spots, pterygostomian spines (Wicksten 2011). giving gray appearance. Rostrum: Rostrum straight and up- General Morphology: The body of decapod turned (Crangon, Kuris and Carlton 1977).