Analysis of Alternatives
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Model of Chromate Leaching from Inhibited Primers
18th World IMACS / MODSIM Congress, Cairns, Australia 13-17 July 2009 http://mssanz.org.au/modsim09 A model of chromate leaching from inhibited primers Jenkins, D.R. 1 and A.D. Miller 2 1 CSIRO Mathematical and Information Sciences, Locked Bag 17, North Ryde NSW 1670 2 CSIRO Mathematical and Information Sciences, Private Bag No 2, Glen Osmond SA 5064 Email: [email protected] Abstract: We present a model of the production, transport and reaction of various ionic species involved in the process of leaching of chromate ions from primer layers on metal substrates. The polymer primer layers contain a range of filler particles, some of which are inert, while others are “active”, in the sense that they act to inhibit the onset and progression of corrosion in bare metal, in a situation where the primer layer is breached. A key active corrosion inhibitor is chromate ions, usually provided by strontium chromate (SrCrO4) particles in the primer. The chromate ions are formed by dissolution in water, which needs to penetrate the primer layer for this to occur. The ions then leach out to the damage site and react with the metal surface to inhibit corrosion. Unfortunately chromate is carcinogenic and therefore is now banned in various parts of the world. More acceptable alternatives for chromate are being keenly sought. A valuable aspect of chromate ions in their inhibitor role is their ability to relatively rapidly leach out after damage initiation, which is not true of many other inhibitors. However, aspects of the mechanism of chromate leaching through primer layers are not well understood. -

Strontium Chromate from Austria and France
Strontium Chromate from Austria and France Investigation Nos. 731-TA-1422-1423 (Preliminary) Publication 4836 October 2018 U.S. International Trade Commission Washington, DC 20436 U.S. International Trade Commission COMMISSIONERS David S. Johanson, Chairman Irving A. Williamson Meredith M. Broadbent Rhonda K. Schmidtlein Jason E. Kearns Catherine DeFilippo Director of Operations Staff assigned Kristina Lara, Investigator Samantha DeCarlo, Industry Analyst Tana von Kessler Economist Jennifer Brinckhaus, Accountant KaDeadra McNealy, Statistician David Goldfine, Attorney Douglas Corkran, Supervisory Investigator Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Strontium Chromate from Austria and France Investigation Nos. 731-TA-1422-1423 (Preliminary) Publication 4836 October 2018 CONTENTS Page ....................................................................................................................... 1 ........................................................................................................ 3 Introduction ................................................................................................................ I‐1 Background ................................................................................................................................ I‐1 Statutory criteria and organization of the report .................................................................... -

DECOMPOSITION of MIXTURES of Strontlum CHROMATE and STRONTIUM CARBONATE
PART V!. DECOMPOSITION OF MIXTURES OF STRONTlUM CHROMATE AND STRONTIUM CARBONATE. By V. T. Atlzaz~alealzd S. K. K. Jatkar. INTRBDUCTIQN. It has been shown in Part V (This Jo~trnal, 1938, ZlA, 159) that the decon~positionof strontium chromate indicated intermediate stages at 50, 66.6 and 75% clecomposition in conformity with our observations on the decomposition of calcium chromate (This Jownal, 1937, ZOA, 55-56). The stages corresponding- to 80 and 100% deconlposition could not be obtained in this case because of the lack of a suitable tube material which woulcl stand temperatures above 1450" in vacuum. While studying the decomposition of the mixtures of lime with calcium chromate (This Joz~mal, 21A, 119) two additional stages in the decomposition corresponding to 33.3 and 40% clecom- position were obtained, the composition of each stage being, respec- tively, identical with the acid-soluble portions from 66.6 and 75 % stages for plain chromate. The composition of the acid-soluble por- tion is thus an indication of the con~position of the intermediate stages obtained in the decomposition of the chromate when mixed with the corresponcling oxide. The difference in the behaviour of the con~poundsformed at the 50% stages for calcium and strontium chronlates towards acid has already been pointed out in Part V of this series. We thus get from 50% stage For strontium chromate, an acid-soluble compound 8Sr0 4Cr03 Cr,O, corresi~onclingto 33.3% decomposition, which though obtained otherwise in the decomposition of calcium chromate and its mixtures with lime, contained one mot of SrO less, ihile the corres- ponding stage in the decomposition of calcium chromate yields an acirl-soluble portion 10Ca0 6Cr0, Cr,O, A strontium compound corresponding to 33.370 stage was also obtained by treating the 66.6% stage with acid, the composition of which was 9Sr0 4Cr0, Cr,O,. -

IAEG® Declaration Development Support Document
IAEG® Declaration Development Support Document 14 July 2021 Version 2 THIS DOCUMENT IS PROVIDED BY INTERNATIONAL AEROSPACE ENVIRONMENTAL GROUP, INC. (“IAEG”) FOR INFORMATIONAL PURPOSES ONLY. ANY INACCURACY OR OMISSION IS NOT THE RESPONSIBILITY OF IAEG. IAEG DOES NOT MAKE ANY REPRESENTATIONS OR WARRANTIES WITH RESPECT TO THIS DOCUMENT OR ITS CONTENTS. IAEG HEREBY DISCLAIMS ALL WARRANTIES OF ANY NATURE, EXPRESS, IMPLIED OR OTHERWISE, OR ARISING FROM TRADE OR CUSTOM, INCLUDING, WITHOUT LIMITATION, ANY IMPLIED WARRANTIES OF MERCHANTABILITY, NONINFRINGEMENT, QUALITY, TITLE, FITNESS FOR A PARTICULAR PURPOSE, COMPLETENESS OR ACCURACY. TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAWS, IAEG SHALL NOT BE LIABLE FOR ANY LOSSES, EXPENSES OR DAMAGES OF ANY NATURE, INCLUDING, WITHOUT LIMITATION, SPECIAL, INCIDENTAL, PUNITIVE, DIRECT, INDIRECT OR CONSEQUENTIAL DAMAGES OR LOST INCOME OR PROFITS, RESULTING FROM OR ARISING OUT OF A COMPANY’S OR INDIVIDUAL’S USE OF THIS DOCUMENT, WHETHER ARISING IN TORT, CONTRACT, STATUTE, OR OTHERWISE, EVEN IF ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. IAEG IS COMMITTED TO COMPLYING WITH ALL LAWS, INCLUDING ANTITRUST LAWS. IAEG PROVIDES THIS DOCUMENT FOR INFORMATIONAL PURPOSES ONLY. DETERMINATION OF WHETHER AND/OR HOW TO USE ALL OR ANY PORTION OF THIS DOCUMENT IS TO BE MADE IN YOUR SOLE AND ABSOLUTE DISCRETION. NO PART OF THIS DOCUMENT CONSTITUTES LEGAL ADVICE OR DIRECTION. PRIOR TO USING THIS DOCUMENT, YOU SHOULD REVIEW IT, ALONG WITH APPLICABLE LAWS AND REGULATIONS, WITH YOUR OWN LEGAL COUNSEL. USE OF THIS DOCUMENT -

Substance Name: Strontium Chromate EC Number: 232-142-6 CAS Number: 7789-06-2
SVHC SUPPORT DOCUMENT – STRONTIUM CHROMATE Substance name: Strontium chromate EC number: 232-142-6 CAS number: 7789-06-2 MEMBER STATE COMMITTEE SUPPORT DOCUMENT FOR IDENTIFICATION OF STRONTIUM CHROMATE AS A SUBSTANCE OF VERY HIGH CONCERN BECAUSE OF ITS CMR PROPERTIES Adopted on 20 May 2011 1 SVHC SUPPORT DOCUMENT – STRONTIUM CHROMATE CONTENTS 1 IDENTITY OF THE SUBSTANCE AND PHYSICAL AND CHEMICAL PROPERTIES .................................4 1.1 Name and other identifiers of the substance ...................................................................................................4 1.2 Composition of the substance.........................................................................................................................5 1.3 Physico-chemical properties...........................................................................................................................6 2 HARMONISED CLASSIFICATION AND LABELLING....................................................................................7 3 ENVIRONMENTAL FATE PROPERTIES...........................................................................................................8 4 HUMAN HEALTH HAZARD ASSESSMENT.....................................................................................................8 5 ENVIRONMENTAL HAZARD ASSESSMENT .................................................................................................8 6 CONCLUSIONS ON THE SVHC PROPERTIES .................................................................................................8 -

Analysis of Alternatives
ANALYSIS OF ALTERNATIVES Legal name of applicants: AKZO Nobel Car Refinishes B.V. Habich GmbH; Henkel Global Supply Chain B.V.; Indestructible Paint Ltd.; Finalin GmbH; Mapaero; PPG Central (UK) Ltd in its legal capacity as Only Representative of PRC DeSoto International Inc. - OR5; PPG Industries (UK) Ltd; PPG Coatings SA; Aviall Services Inc. Submitted by: AKZO Nobel Car Refinishes B.V. Substance: Strontium chromate, EC No: 232-142-6, CAS No: 7789-06-2 Use title: Application of paints, primers and specialty coatings containing Strontium chromate in the construction of aerospace and aeronautical parts, including aeroplanes / helicopters, space craft, satellites, launchers, engines, and for the maintenance of such constructions, as well as for such aerospace and aeronautical parts, used elsewhere, where the supply chain and exposure scenarios are identical. Use number: 2 Use number: 2 Copy right protected - Property of Members of the CCST Consortium - No copying / use allowed. ANALYSIS OF ALTERNATIVES Disclaimer This document shall not be construed as expressly or implicitly granting a license or any rights to use related to any content or information contained therein. In no event shall applicant be liable in this respect for any damage arising out or in connection with access, use of any content or information contained therein despite the lack of approval to do so. ii Use number: 2 Copy right protected - Property of Members of the CCST Consortium - No copying / use allowed. ANALYSIS OF ALTERNATIVES CONTENTS 1. SUMMARY 1 2. INTRODUCTION 8 2.1. Substance 8 2.2. Uses of Cr(VI) containing substances 8 2.3. Purpose and benefits of Cr(VI) compounds 8 3. -

Barium, Zinc and Strontium Yellows in Late 19Th–Early 20Th Century Oil Paintings Vanessa Otero1* , Marta F
Otero et al. Herit Sci (2017) 5:46 DOI 10.1186/s40494-017-0160-3 RESEARCH ARTICLE Open Access Barium, zinc and strontium yellows in late 19th–early 20th century oil paintings Vanessa Otero1* , Marta F. Campos1, Joana V. Pinto2, Márcia Vilarigues1, Leslie Carlyle1 and Maria João Melo1 Abstract This work focuses on the study of the 19th century yellow chromate pigments based on barium (BaCrO 4), zinc (4ZnCrO4 K2O 3H2O) and strontium (SrCrO4). These pigments, which are reported to shift in hue and darken, have been found· in· 19th century artworks. A better understanding of their historic manufacture will contribute to the visual/chemical interpretation of change in these colours. Research was carried out on the Winsor & Newton (W&N) 19th century archive database providing a unique insight into their manufacturing processes. One hundred and three production records were found, 69% for barium, 25% for zinc and 6% for strontium chromates, mainly under the names Lemon, Citron and Strontian Yellow, respectively. Analysis of the records shows that each pigment is charac- terised by only one synthetic pathway. The low number of records found for the production of strontium chromate suggests W&N was not selling this pigment formulation on a large scale. Furthermore, contrary to what the authors have discovered for W&N chrome yellow pigments, extenders were not added to these pigment formulations, most probably due to their lower tinting strength (TS). The latter was calculated in comparison to pure chrome yellow (PbCrO4, 100% TS) resulting in 92% for barium, 65% for zinc potassium and 78% for strontium chromate pigments. -

Strontium Chromate from Austria and France (Final)
Strontium Chromate from Austria and France Investigation Nos. 731-TA-1422 and 731-TA-1423 (Final) Publication 4992 November 2019 U.S. International Trade Commission Washington, DC 20436 U.S. International Trade Commission COMMISSIONERS David S. Johanson, Chairman Rhonda K. Schmidtlein Jason E. Kearns Randolph J. Stayin Amy A. Karpel Catherine DeFilippo Director of Operations Staff assigned Christopher S. Robinson, Investigator Samuel Goodman, Industry Analyst Carlos Payan, Economist Jennifer Brinkhaus, Accountant Maya Alexander, Statistician Henry Smith, Attorney Douglas Corkran, Supervisory Investigator Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Strontium Chromate from Austria and France Investigation Nos. 731-TA-1422 and 731-TA-1423 (Final) Publication 4992 November 2019 CONTENTS Page Determinations ............................................................................................................................... 1 Views of the Commission ............................................................................................................... 3 Part I: Introduction .............................................................................................................. I-1 Background ................................................................................................................................ I-1 Statutory criteria ...................................................................................................................... -

Chromium Hexavalent Compounds
SUBSTANCE PROFILES Chromium Hexavalent Compounds* Additional Information Relevant to Carcinogenicity Known to be human carcinogens Chromosomal aberrations (changes in chromosome structure or First Listed in the First Annual Report on Carcinogens (1980) number), sister chromatid exchange, and aneuploidy (extra or missing Carcinogenicity chromosomes) were observed in workers exposed to chromium(VI) compounds. Chromium(VI) compounds also caused genetic damage Chromium hexavalent (VI) compounds are known to be human in a variety of test systems. Most caused mutations and DNA damage carcinogens based on sufficient evidence of carcinogenicity in humans. in bacteria; however, the poorly soluble compounds had to be Epidemiological studies in various geographical locations have dissolved in acids or alkalies to produce genetic effects. A few consistently reported increased risks of lung cancer among workers compounds also caused mutations in yeast and insects. Many engaged in chromate production, chromate pigment production, and chromium(VI) compounds caused genetic damage in cultured human chromium plating. Epidemiological studies of lung cancer among and other animal cells and in experimental animals exposed in vivo. ferrochromium workers were inconclusive. Exposure to specific The compounds tested included ammonium chromate and chromium compounds varies by industry. Chromate production workers dichromate, calcium chromate, chromium trioxide, sodium chromate are exposed to a variety of chromium compounds, including and dichromate, potassium chromate and dichromate, strontium chromium(VI) and trivalent (III) compounds. Chromate pigment chromate, and the industrial product basic zinc chromate (zinc workers are exposed to chromates in the pigment and to soluble yellow). Among the types of genetic damage observed were gene chromium(VI) compounds used in pigment production. -

WO 2016/040564 Al 17 March 2016 (17.03.2016) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2016/040564 Al 17 March 2016 (17.03.2016) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every C05F 11/00 (2006.01) C05D 9/02 (2006.01) kind of national protection available): AE, AG, AL, AM, C05G 3/00 (2006.01) AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (21) Number: International Application DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, PCT/US20 15/049323 HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (22) International Filing Date: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, 10 September 2015 (10.09.201 5) MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, (25) Filing Language: English SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, (26) Publication Language: English TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (30) Priority Data: (84) Designated States (unless otherwise indicated, for every 62/049,399 12 September 2014 (12.09.2014) US kind of regional protection available): ARIPO (BW, GH, GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, (71) Applicant: SUN CHEMICAL CORPORATION TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, [US/US]; 35 Waterview Boulevard, Parsippany, NJ 07054 TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, (US). -
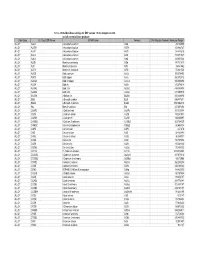
Database Full Listing
16-Nov-06 OLI Data Base Listings for ESP version 7.0.46, Analyzers 2.0.46 and all current alliance products Data Base OLI Tag (ESP) Name IUPAC Name Formula CAS Registry Number Molecular Weight ALLOY AL2U 2-Aluminum uranium Al2U 291.98999 ALLOY AL3TH 3-Aluminum thorium Al3Th 312.982727 ALLOY AL3TI 3-Aluminum titanium Al3Ti 128.824615 ALLOY AL3U 3-Aluminum uranium Al3U 318.971527 ALLOY AL4U 4-Aluminum uranium Al4U 345.953064 ALLOY ALSB Aluminum antimony AlSb 148.731537 ALLOY ALTI Aluminum titanium AlTi 74.861542 ALLOY ALTI3 Aluminum 3-titanium AlTi3 170.621536 ALLOY AUCD Gold cadmium AuCd 309.376495 ALLOY AUCU Gold copper AuCu 260.512512 ALLOY AUCU3 Gold 3-copper AuCu3 387.604492 ALLOY AUSN Gold tin AuSn 315.676514 ALLOY AUSN2 Gold 2-tin AuSn2 434.386505 ALLOY AUSN4 Gold 4-tin AuSn4 671.806519 ALLOY BA2SN 2-Barium tin Ba2Sn 393.369995 ALLOY BI2U 2-Bismuth uranium Bi2U 655.987671 ALLOY BI4U3 4-Bismuth 3-uranium Bi4U3 1550.002319 ALLOY BIU Bismuth uranium BiU 447.007294 ALLOY CA2PB 2-Calcium lead Ca2Pb 287.355988 ALLOY CA2SI 2-Calcium silicon Ca2Si 108.241501 ALLOY CA2SN 2-Calcium tin Ca2Sn 198.865997 ALLOY CA3SB2 3-Calcium 2-antimony Ca3Sb2 363.734009 ALLOY CAMG2 Calcium 2-magnesium CaMg2 88.688004 ALLOY CAPB Calcium lead CaPb 247.278 ALLOY CASI Calcium silicon CaSi 68.163498 ALLOY CASI2 Calcium 2-silicon CaSi2 96.249001 ALLOY CASN Calcium tin CaSn 158.787994 ALLOY CAZN Calcium zinc CaZn 105.468002 ALLOY CAZN2 Calcium 2-zinc CaZn2 170.858002 ALLOY CD11U 11-Cadmium uranium Cd11U 1474.536865 ALLOY CD3AS2 3-Cadmium 2-arsenic As2Cd3 487.073212 -

United States Patent (19) (11) 4,138,477 Gaffar 45 Feb
United States Patent (19) (11) 4,138,477 Gaffar 45 Feb. 6, 1979 (54) COMPOSITION TO CONTROL MOUTH 3,956,480 5/1976 Dichter .................................. 424/49 ODOR 4,022,880 5/1977 Vinson ................................... 424/49 75) Inventor: Maria Corazon S. Gaffar, Somerset, OTHER PUBLICATIONS Websters Third New International Dictionary (un (73) Assignee: Colgate Palmolive Company, New abridged) 1963, p. 2174, "Sorbic Acid and/or Sorbate.” York, N.Y. Crisp et al., "Zinc Polycarboxylate Cements:' J. Dent res. 1976, 55, 2, pp. 299-308. (21) Appl. No.: 691,262 Begala et al., J. of Physical Chem., (1972), 76, 2, pp. 22 Filed: May 28, 1976 254-260. 51) Int. Cl.’......................... A61K 7/16; A61K 7/18; Primary Examiner-Shep K. Rose A61K9/68; A61K 31/315 Attorney, Agent, or Firm-Robert L. Stone; Murray M. 52 U.S. C. ........................................ 424/52; 424/48; Grill; Herbert S. Sylvester 424/49; 424/56; 424/78; 424/81; 424/83; 424/145; 424/289 57 ABSTRACT 58) Field of Search .................................... 424/48-58, A novel composition to prevent and control mouth 424/78-83, 145, 289 odor, which is also effective in preventing calculus, (56) References Cited plaque, caries and periodontal disease containing as the U.S. PATENT DOCUMENTS essential agent, a zinc-polymer combination formed by the reaction or interaction of a zinc compound with an 1,593,485 7/1926 Crosnier ................................. 424/76 anionic polymer containing carboxylic, sulfonic and/or 3,070,510 12/1962 Cooley .................. ... 424/52 phosphonic acid radicals. 3,429,963 2/1969 Shedlovsky et al ... 424/56 3,943,267 3/1976 Randol ..................