Screening Assessment for the Challenge C.I. Pigment Yellow 34
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
RAL Colour Chart
RAL Colour Chart The colours depicted on the following chart are for guidelines only. The finished colour may not be as shown here. 1000 1001 1002 1003 1004 1005 Green Beige Pale Beige Sand Yellow Signal Yellow Dark Golden Honey Yellow Yellow 1006 1007 1011 1012 1013 1014 Maize Yellow Chrome Yellow Brown Beige Lemon Yellow Pearl White Dark Ivory 1015 1016 1017 1018 1019 1020 Light Ivory Sulphur Yellow Saffron Yellow Zinc Yellow Grey Beige Olive Yellow 1021 1023 1024 1027 1028 1032 Cadmium Yellow Traffic Yellow Ochre Yellow Curry Yellow Mellon Yellow Broom Yellow 1033 1034 2000 2001 2002 2003 Dahlia Yellow Pastel Yellow Yellow Orange Red Orange Vermillion Pastel Orange 2004 2008 2009 2010 2011 2012 Pure Orange Light Red Traffic Orange Signal Orange Deep Orange Salmon Orange Orange 3000 3001 3002 3003 3004 3005 Flame Red RAL Signal Red Carmine Red Ruby Red Purple Red Wine Red 3007 3009 3011 3012 3013 3014 Black Red Oxide Red Brown Red Beige Red Tomato Red Antique Pink 3015 3016 3017 3018 3020 3022 Light Pink Coral Red Rose Strawberry Red Traffic Red Dark Salmon Red 3027 3031 4001 4002 4003 4004 Raspberry Red Orient Red Red Lilac Red Violet Heather Violet Claret Violet 4005 4006 4007 4008 4009 4010 Blue Lilac Traffic Purple Purple Violet Signal Violet Pastel Violet Telemagenta 5000 5001 5002 5003 5004 5005 Violet Blue Green Blue Ultramarine Blue dark Sapphire Black Blue Signal Blue Blue 5007 5008 5009 5010 5011 5012 Brilliant Blue Grey Blue Light Azure Blue Gentian Blue Steel Blue Light Blue 5013 5014 5015 5017 5018 5019 Dark Cobalt Blue -

Watercolours: What´S Different in the New Assortment?
Product information Watercolours HORADAM® watercolours: What´s different in the new assortment? The following colours have got a new name: Art.-No. Old Name New Name 14 208 Aureolin modern Aureolin hue 14 209 Translucent yellow Transparent Yellow 14 211 Chrome yellow lemon Chromium yellow hue lemon 14 212 Chrome yellow light Chromium yellow hue light 14 213 Chrome yellow deep Chromium yellow hue deep 14 214 Chrome orange Chromium orange hue 14 218 Translucent orange Transparent orange 14 366 Deep red Perylene maroon 14 476 Mauve Schmincke violet 14 498 Dark blue indigo Dark blue 14 648 Sepia brown tone Sepia brown reddish 14 786 Charcoal grey Anthracite The following colours are completely omitted due to raw materials which aren’t available anymore: Art.-No. Old Name 14 652 Walnut brown This tone cannot be mixed out of other colours. 14 666 Pozzuoli earth This tone can be mixed using 14 649 English Venetian red and the slightly reddish 14 670 Madder brown. These colours are now produced using other pigments. Therefore they have got a new name and a new number: Old Art.-No. Old Name New Art.-No. New Name 14 345 Dark red 14 344 Perylene dark red 14 210 Gamboge gum modern 14 217 Quinacridone gold hue 14 478 Helio blue reddish 14 477 Phthalo sapphire blue 14 536 Green yellow 14 537 Transparent green gold - Discontinued colours The described product attributes and application examples have been tes- a warranty for product attributes and/or assume liability for damages that ted in the Schmincke laboratory. -

Derivation of Proposed 2007 Draft Matrix Soil Standards for Barium
Derivation of Proposed 2007 Draft Matrix Soil Standards for Barium Glyn R. Fox Environmental Management Branch Environmental Protection Division September 24, 2007 Victoria, British Columbia Table of Contents Page 1. Introduction ……………………………………………………………………… 4 2. Details Related to Derivation of Proposed 2007 Draft Matrix Soil Standards 2.1 Derivation of Human Health Protection Standards – Intake of contaminated soil ………………………………………………………….. 7 2.2 Derivation of Human health Protection Standards – Groundwater used for drinking water ……………………………….…………….......... 7 2.3 Derivation of Environmental Protection Standards – Toxicity to soil invertebrates and plants ……………………………………….......... 9 2.4 Derivation of Environmental Protection Standards – Livestock ingesting soil and fodder ………………………………………………… 9 2.5 Derivation of Environmental Protection Standards – Major microbial function impairment ………………………………….……… 10 2.6 Derivation of Environmental Protection Standards – Groundwater flow to surface water used by aquatic life ..…………………………… 10 2.7 Derivation of Environmental Protection Standards – Groundwater used for livestock watering ……………………………………………… 11 2.8 Derivation of Environmental Protection Standards – Groundwater used for irrigation ………………………………………………………… 12 2.9 CSST “Background” Adjustment ……………………..……………… 12 2.10 Application of CSST Rounding-off Rule ……………………………… 12 3. References …….…………………………………………………………………… 13 2 Table of Contents (continued) Page 4. Exhibits Exhibit 1. Proposed 2007 Draft Matrix Soil Standards for Barium ……..… 19 5. Tables -

RAL COLOR CHART ***** This Chart Is to Be Used As a Guide Only. Colors May Appear Slightly Different ***** Green Beige Purple V
RAL COLOR CHART ***** This Chart is to be used as a guide only. Colors May Appear Slightly Different ***** RAL 1000 Green Beige RAL 4007 Purple Violet RAL 7008 Khaki Grey RAL 4008 RAL 7009 RAL 1001 Beige Signal Violet Green Grey Tarpaulin RAL 1002 Sand Yellow RAL 4009 Pastel Violet RAL 7010 Grey RAL 1003 Signal Yellow RAL 5000 Violet Blue RAL 7011 Iron Grey RAL 1004 Golden Yellow RAL 5001 Green Blue RAL 7012 Basalt Grey Ultramarine RAL 1005 Honey Yellow RAL 5002 RAL 7013 Brown Grey Blue RAL 1006 Maize Yellow RAL 5003 Saphire Blue RAL 7015 Slate Grey Anthracite RAL 1007 Chrome Yellow RAL 5004 Black Blue RAL 7016 Grey RAL 1011 Brown Beige RAL 5005 Signal Blue RAL 7021 Black Grey RAL 1012 Lemon Yellow RAL 5007 Brillant Blue RAL 7022 Umbra Grey Concrete RAL 1013 Oyster White RAL 5008 Grey Blue RAL 7023 Grey Graphite RAL 1014 Ivory RAL 5009 Azure Blue RAL 7024 Grey Granite RAL 1015 Light Ivory RAL 5010 Gentian Blue RAL 7026 Grey RAL 1016 Sulfer Yellow RAL 5011 Steel Blue RAL 7030 Stone Grey RAL 1017 Saffron Yellow RAL 5012 Light Blue RAL 7031 Blue Grey RAL 1018 Zinc Yellow RAL 5013 Cobolt Blue RAL 7032 Pebble Grey Cement RAL 1019 Grey Beige RAL 5014 Pigieon Blue RAL 7033 Grey RAL 1020 Olive Yellow RAL 5015 Sky Blue RAL 7034 Yellow Grey RAL 1021 Rape Yellow RAL 5017 Traffic Blue RAL 7035 Light Grey Platinum RAL 1023 Traffic Yellow RAL 5018 Turquiose Blue RAL 7036 Grey RAL 1024 Ochre Yellow RAL 5019 Capri Blue RAL 7037 Dusty Grey RAL 1027 Curry RAL 5020 Ocean Blue RAL 7038 Agate Grey RAL 1028 Melon Yellow RAL 5021 Water Blue RAL 7039 Quartz Grey -

A Model of Chromate Leaching from Inhibited Primers
18th World IMACS / MODSIM Congress, Cairns, Australia 13-17 July 2009 http://mssanz.org.au/modsim09 A model of chromate leaching from inhibited primers Jenkins, D.R. 1 and A.D. Miller 2 1 CSIRO Mathematical and Information Sciences, Locked Bag 17, North Ryde NSW 1670 2 CSIRO Mathematical and Information Sciences, Private Bag No 2, Glen Osmond SA 5064 Email: [email protected] Abstract: We present a model of the production, transport and reaction of various ionic species involved in the process of leaching of chromate ions from primer layers on metal substrates. The polymer primer layers contain a range of filler particles, some of which are inert, while others are “active”, in the sense that they act to inhibit the onset and progression of corrosion in bare metal, in a situation where the primer layer is breached. A key active corrosion inhibitor is chromate ions, usually provided by strontium chromate (SrCrO4) particles in the primer. The chromate ions are formed by dissolution in water, which needs to penetrate the primer layer for this to occur. The ions then leach out to the damage site and react with the metal surface to inhibit corrosion. Unfortunately chromate is carcinogenic and therefore is now banned in various parts of the world. More acceptable alternatives for chromate are being keenly sought. A valuable aspect of chromate ions in their inhibitor role is their ability to relatively rapidly leach out after damage initiation, which is not true of many other inhibitors. However, aspects of the mechanism of chromate leaching through primer layers are not well understood. -
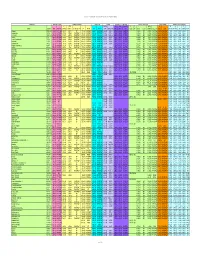
Chemical-Specific Parameters Supporting Table May 2016 Analyte
Regional Screening Level (RSL) Chemical-specific Parameters Supporting Table May 2016 Contaminant Molecular Weight Volatility Parameters Melting Point Density Diffusivity in Air and Water Partition Coefficients Water Solubility Tapwater Dermal Parameters H` (atm- Density Dia Diw Dia and Diw Kd Kd Koc log Kow S B τevent t* Kp 3 3 2 2 Analyte CAS No. MW MW Ref (unitless) m /mole) H` and HLC Ref VP VP Ref MP MP Ref (g/cm ) Density Ref (cm /s) (cm /s) Ref (L/kg) Ref (L/kg) Koc Ref (unitless) log Kow Ref (mg/L) S Ref (unitless) (hr/event) (hr) (cm/hr) K Ref Acephate 30560-19-1 1.8E+02 PHYSPRO 2.0E-11 5.0E-13 EPI 1.7E-06 PHYSPROP 8.8E+01 PHYSPROP 1.4E+00 CRC89 3.7E-02 8.0E-06 WATER9 1.0E+01 EPI -8.5E-01 PHYSPRO 8.2E+05 PHYSPROP 2.1E-04 1.1E+00 2.7E+00 4.0E-05 EPI Acetaldehyde 75-07-0 4.4E+01 PHYSPRO 2.7E-03 6.7E-05 PHYSPROP 9.0E+02 PHYSPROP -1.2E+02 PHYSPROP 7.8E-01 CRC89 1.3E-01 1.4E-05 WATER9 1.0E+00 EPI -3.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.3E-03 1.9E-01 4.5E-01 5.3E-04 EPI Acetochlor 34256-82-1 2.7E+02 PHYSPRO 9.1E-07 2.2E-08 PHYSPROP 2.8E-05 PHYSPROP 1.1E+01 PubChem 1.1E+00 PubChem 2.2E-02 5.6E-06 WATER9 3.0E+02 EPI 3.0E+00 PHYSPRO 2.2E+02 PHYSPROP 3.1E-02 3.4E+00 8.2E+00 5.0E-03 EPI Acetone 67-64-1 5.8E+01 PHYSPRO 1.4E-03 3.5E-05 PHYSPROP 2.3E+02 PHYSPROP -9.5E+01 PHYSPROP 7.8E-01 CRC89 1.1E-01 1.2E-05 WATER9 2.4E+00 EPI -2.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.5E-03 2.2E-01 5.3E-01 5.1E-04 EPI Acetone Cyanohydrin 75-86-5 8.5E+01 PHYSPRO 8.1E-08 2.0E-09 PHYSPROP 3.4E-01 PHYSPROP -1.9E+01 PHYSPROP 9.3E-01 CRC89 8.6E-02 1.0E-05 WATER9 1.0E+00 -

Strontium Chromate from Austria and France
Strontium Chromate from Austria and France Investigation Nos. 731-TA-1422-1423 (Preliminary) Publication 4836 October 2018 U.S. International Trade Commission Washington, DC 20436 U.S. International Trade Commission COMMISSIONERS David S. Johanson, Chairman Irving A. Williamson Meredith M. Broadbent Rhonda K. Schmidtlein Jason E. Kearns Catherine DeFilippo Director of Operations Staff assigned Kristina Lara, Investigator Samantha DeCarlo, Industry Analyst Tana von Kessler Economist Jennifer Brinckhaus, Accountant KaDeadra McNealy, Statistician David Goldfine, Attorney Douglas Corkran, Supervisory Investigator Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Strontium Chromate from Austria and France Investigation Nos. 731-TA-1422-1423 (Preliminary) Publication 4836 October 2018 CONTENTS Page ....................................................................................................................... 1 ........................................................................................................ 3 Introduction ................................................................................................................ I‐1 Background ................................................................................................................................ I‐1 Statutory criteria and organization of the report .................................................................... -

A Short History of 18-19Th Century
A SHORT HISTORY OF 18-19TH CENTURY BRITISH HAND-COLOURED PRINTS; WITH A FOCUS ON GAMBOGE, CHROME YELLOW AND QUERCITRON; THEIR SENSITIVITIES AND THEIR IMPACT ON AQUEOUS CONSERVATION TREATMENTS Stacey Mei Kelly (13030862) A Dissertation presented at Northumbria University for the degree of MA in Conservation of Fine Art, 2015 VA0742 Page 1 of 72 Table of Contents List of figures……………………………………………………………………………………...…2 List of tables……………………………………………………………………………………….....2 Abstract………………………………………………………………………………………………3 Introduction……………………………………………………………………………..………...…3 Research aims, methodology and resources………………………………………………….…....4 1. Aims……………………………………………………………………………………..…...4 2. Research Questions…………………………………………………………………..…...….4 3. Literature review……………………………………………………………………….....….5 4. Case Study Survey………………………………………………………………………..….5 5. Empirical Work……………………………………………………………………………....6 Chapter 1: A Brief History of Hand-coloured Prints in Britain………………………………....6 1.1 The popularity of hand-coloured prints…………………………………………………….....6 1.2 The people behind hand-colouring……………………………………………………..….….9 1.3 Materials and Methods……………………………………………………………………....14 Chapter 2: Yellow Pigments: A focus on Gamboge, Chrome Yellow, and Quercitron……….19 2.1 Why Gamboge, Chrome Yellow, and Quercitron……………………………………….…..19 2.2 Gamboge……………………………………………………………………………….....….20 2.2.1 History…………………………………………………………………………….…....20 2.2.2 Working properties…………………………………………………………………..…21 2.2.3 Physical and chemical properties…………………………………………………..…..21 2.2.4 Methods of -

DECOMPOSITION of MIXTURES of Strontlum CHROMATE and STRONTIUM CARBONATE
PART V!. DECOMPOSITION OF MIXTURES OF STRONTlUM CHROMATE AND STRONTIUM CARBONATE. By V. T. Atlzaz~alealzd S. K. K. Jatkar. INTRBDUCTIQN. It has been shown in Part V (This Jo~trnal, 1938, ZlA, 159) that the decon~positionof strontium chromate indicated intermediate stages at 50, 66.6 and 75% clecomposition in conformity with our observations on the decomposition of calcium chromate (This Jownal, 1937, ZOA, 55-56). The stages corresponding- to 80 and 100% deconlposition could not be obtained in this case because of the lack of a suitable tube material which woulcl stand temperatures above 1450" in vacuum. While studying the decomposition of the mixtures of lime with calcium chromate (This Joz~mal, 21A, 119) two additional stages in the decomposition corresponding to 33.3 and 40% clecom- position were obtained, the composition of each stage being, respec- tively, identical with the acid-soluble portions from 66.6 and 75 % stages for plain chromate. The composition of the acid-soluble por- tion is thus an indication of the con~position of the intermediate stages obtained in the decomposition of the chromate when mixed with the corresponcling oxide. The difference in the behaviour of the con~poundsformed at the 50% stages for calcium and strontium chronlates towards acid has already been pointed out in Part V of this series. We thus get from 50% stage For strontium chromate, an acid-soluble compound 8Sr0 4Cr03 Cr,O, corresi~onclingto 33.3% decomposition, which though obtained otherwise in the decomposition of calcium chromate and its mixtures with lime, contained one mot of SrO less, ihile the corres- ponding stage in the decomposition of calcium chromate yields an acirl-soluble portion 10Ca0 6Cr0, Cr,O, A strontium compound corresponding to 33.370 stage was also obtained by treating the 66.6% stage with acid, the composition of which was 9Sr0 4Cr0, Cr,O,. -

Examination of Pigments on Thai Manuscripts: the first Identification of Copper Citrate
JOURNAL OF RAMAN SPECTROSCOPY J. Raman Spectrosc. 2008; 39: 1057–1065 Published online 27 May 2008 in Wiley InterScience (www.interscience.wiley.com) DOI: 10.1002/jrs.1985 Examination of pigments on Thai manuscripts: the first identification of copper citrate Katherine Eremin,1∗ Jens Stenger,1 Jo-Fan Huang,3 Alan Aspuru-Guzik,2 Theodore Betley,2 Leslie Vogt,2 Ivan Kassal,2 Scott Speakman4 and Narayan Khandekar1 1 Harvard University Art Museums, 32 Quincy Street, Cambridge, MA 02138, USA 2 Department of Chemistry and Chemical Biology, 12 Oxford Street, Harvard University, Cambridge, MA 02138, USA 3 Philadelphia Museum of Art, Benjamin Franklin Parkway, Philadelphia, PA 19130, USA 4 Center for Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Avenue, Cambridge, MA 02139, USA Received 13 December 2007; Accepted 5 March 2008 Samples from Thai manuscripts dated to the 18th to 20th century were analyzed by Raman spectroscopy and Fourier-transform infrared spectroscopy (FTIR) to determine the pigments used. This suggested a change in palette from the 18th to 20th century, with use of imported pigments in the later manuscripts. In the 18th century, the main green used was an organic copper salt, which was replaced by emerald green and mixtures of Prussian blue with gamboge, chrome yellow and zinc yellow (zinc potassium chromate). Chrome yellow was used in addition to gamboge in one later 19th century manuscript. Similarly, indigo in the 18th century manuscripts was replaced by Prussian blue and then synthetic ultramarine in the 19th century manuscripts. Lead white was the main white pigment in all but one manuscript, which contained huntite, a magnesium calcium carbonate. -

Massachusetts Chemical Fact Sheet
Massachusetts Chemical Fact Sheet Hexavalent Chromium Table 1: HEXAVALENT CHROMIUM COMPOUNDS: Compounds SELECTED EXAMPLES* Compound Chemical Formula CAS # This fact sheet is part of a series of chemical fact sheets Ammonium chromate (NH ) Cr0 7788-98-9 developed by TURI to help Massachusetts companies, 4 2 4 community organizations and residents understand the Ammonium dichromate (NH4)2Cr2O7 7789-09-5 chemical’s use and health and environmental effects, as Barium chromate BaCrO4 10294-40-3 well as the availability of safer alternatives. tert-Butyl Chromate [(CH3)3CO]2CrO2 1189-85-1 Hexavalent chromium compounds are a toxic form of Calcium chromate CaCrO4 13765-19-0 chromium and are used in a variety of industrial processes Chromic acid H2CrO4 7738-94-5 and products. Chromium VI chloride CrCl6 14986-48-2 Hexavalent chromium compounds are human carcinogens, Chromic trioxide CrO3 1333-82-0 mutagens and developmental toxicants and are acutely Hexavalent chromium ion Cr6+ 18540-29-9 toxic. Non-hexavalent chromium compounds do not pose Lead chromate PbCrO4 7758-97-6 the same level of concern with regard to either chronic or Lead chromate oxide PbCrO4-PbO 8454-12-1 acute toxicity. Potassium chlorochromate KCrO3Cl 16037-50-6 Until 2011, all chromium compounds were treated as Potassium chromate K2CrO4 7789-00-6 a single category under TURA. Beginning with Potassium dichromate K Cr O 7778-50-9 reporting year 2012, hexavalent chromium 2 2 7 compounds are reportable under TURA as a Silver chromate Ag2CrO4 7784-01-2 separate category and are designated as a Higher Sodium chromate Na2CrO4 7775-11-3 Hazard Substance, which lowers the reporting Sodium dichromate 7789-12-0 threshold to 1,000 lb/year. -

20210311 IAEG AD-DSL V5.0 for Pdf.Xlsx
IAEGTM AD-DSL Release Version 4.1 12-30-2020 Authority: IAEG Identity: AD-DSL Version number: 4.1 Issue Date: 2020-12-30 Key Yellow shading indicates AD-DSL family group entries, which can be expanded to display a non-exhaustive list of secondary CAS numbers belonging to the family group Substance Identification Change Log IAEG Regulatory Date First Parent Group IAEG ID CAS EC Name Synonyms Revision Date ECHA ID Entry Type Criteria Added IAEG ID IAEG000001 1327-53-3 215-481-4 Diarsenic trioxide Arsenic trioxide R1;R2;D1 2015-03-17 2015-03-17 100.014.075 Substance Direct Entry IAEG000002 1303-28-2 215-116-9 Diarsenic pentaoxide Arsenic pentoxide; Arsenic oxide R1;R2;D1 2015-03-17 2015-03-17 100.013.743 Substance Direct Entry IAEG000003 15606-95-8 427-700-2 Triethyl arsenate R1;R2;D1 2015-03-17 2017-08-14 100.102.611 Substance Direct Entry IAEG000004 7778-39-4 231-901-9 Arsenic acid R1;R2;D1 2015-03-17 2015-03-17 100.029.001 Substance Direct Entry IAEG000005 3687-31-8 222-979-5 Trilead diarsenate R1;R2;D1 2015-03-17 2017-08-14 100.020.890 Substance Direct Entry IAEG000006 7778-44-1 231-904-5 Calcium arsenate R1;R2;D1 2015-03-17 2017-08-14 100.029.003 Substance Direct Entry IAEG000009 12006-15-4 234-484-1 Cadmium arsenide Tricadmium diarsenide R1;R2;D1 2017-08-14 2017-08-14 Substance Direct Entry IAEG000021 7440-41-7 231-150-7 Beryllium (Be) R2 2015-03-17 2019-01-24 Substance Direct Entry IAEG000022 1306-19-0 215-146-2 Cadmium oxide R1;R2;D1 2015-03-17 2017-08-14 100.013.770 Substance Direct Entry IAEG000023 10108-64-2 233-296-7 Cadmium