Substance Name: Strontium Chromate EC Number: 232-142-6 CAS Number: 7789-06-2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Derivation of Proposed 2007 Draft Matrix Soil Standards for Barium
Derivation of Proposed 2007 Draft Matrix Soil Standards for Barium Glyn R. Fox Environmental Management Branch Environmental Protection Division September 24, 2007 Victoria, British Columbia Table of Contents Page 1. Introduction ……………………………………………………………………… 4 2. Details Related to Derivation of Proposed 2007 Draft Matrix Soil Standards 2.1 Derivation of Human Health Protection Standards – Intake of contaminated soil ………………………………………………………….. 7 2.2 Derivation of Human health Protection Standards – Groundwater used for drinking water ……………………………….…………….......... 7 2.3 Derivation of Environmental Protection Standards – Toxicity to soil invertebrates and plants ……………………………………….......... 9 2.4 Derivation of Environmental Protection Standards – Livestock ingesting soil and fodder ………………………………………………… 9 2.5 Derivation of Environmental Protection Standards – Major microbial function impairment ………………………………….……… 10 2.6 Derivation of Environmental Protection Standards – Groundwater flow to surface water used by aquatic life ..…………………………… 10 2.7 Derivation of Environmental Protection Standards – Groundwater used for livestock watering ……………………………………………… 11 2.8 Derivation of Environmental Protection Standards – Groundwater used for irrigation ………………………………………………………… 12 2.9 CSST “Background” Adjustment ……………………..……………… 12 2.10 Application of CSST Rounding-off Rule ……………………………… 12 3. References …….…………………………………………………………………… 13 2 Table of Contents (continued) Page 4. Exhibits Exhibit 1. Proposed 2007 Draft Matrix Soil Standards for Barium ……..… 19 5. Tables -

A Model of Chromate Leaching from Inhibited Primers
18th World IMACS / MODSIM Congress, Cairns, Australia 13-17 July 2009 http://mssanz.org.au/modsim09 A model of chromate leaching from inhibited primers Jenkins, D.R. 1 and A.D. Miller 2 1 CSIRO Mathematical and Information Sciences, Locked Bag 17, North Ryde NSW 1670 2 CSIRO Mathematical and Information Sciences, Private Bag No 2, Glen Osmond SA 5064 Email: [email protected] Abstract: We present a model of the production, transport and reaction of various ionic species involved in the process of leaching of chromate ions from primer layers on metal substrates. The polymer primer layers contain a range of filler particles, some of which are inert, while others are “active”, in the sense that they act to inhibit the onset and progression of corrosion in bare metal, in a situation where the primer layer is breached. A key active corrosion inhibitor is chromate ions, usually provided by strontium chromate (SrCrO4) particles in the primer. The chromate ions are formed by dissolution in water, which needs to penetrate the primer layer for this to occur. The ions then leach out to the damage site and react with the metal surface to inhibit corrosion. Unfortunately chromate is carcinogenic and therefore is now banned in various parts of the world. More acceptable alternatives for chromate are being keenly sought. A valuable aspect of chromate ions in their inhibitor role is their ability to relatively rapidly leach out after damage initiation, which is not true of many other inhibitors. However, aspects of the mechanism of chromate leaching through primer layers are not well understood. -
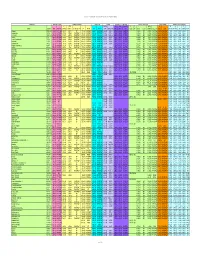
Chemical-Specific Parameters Supporting Table May 2016 Analyte
Regional Screening Level (RSL) Chemical-specific Parameters Supporting Table May 2016 Contaminant Molecular Weight Volatility Parameters Melting Point Density Diffusivity in Air and Water Partition Coefficients Water Solubility Tapwater Dermal Parameters H` (atm- Density Dia Diw Dia and Diw Kd Kd Koc log Kow S B τevent t* Kp 3 3 2 2 Analyte CAS No. MW MW Ref (unitless) m /mole) H` and HLC Ref VP VP Ref MP MP Ref (g/cm ) Density Ref (cm /s) (cm /s) Ref (L/kg) Ref (L/kg) Koc Ref (unitless) log Kow Ref (mg/L) S Ref (unitless) (hr/event) (hr) (cm/hr) K Ref Acephate 30560-19-1 1.8E+02 PHYSPRO 2.0E-11 5.0E-13 EPI 1.7E-06 PHYSPROP 8.8E+01 PHYSPROP 1.4E+00 CRC89 3.7E-02 8.0E-06 WATER9 1.0E+01 EPI -8.5E-01 PHYSPRO 8.2E+05 PHYSPROP 2.1E-04 1.1E+00 2.7E+00 4.0E-05 EPI Acetaldehyde 75-07-0 4.4E+01 PHYSPRO 2.7E-03 6.7E-05 PHYSPROP 9.0E+02 PHYSPROP -1.2E+02 PHYSPROP 7.8E-01 CRC89 1.3E-01 1.4E-05 WATER9 1.0E+00 EPI -3.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.3E-03 1.9E-01 4.5E-01 5.3E-04 EPI Acetochlor 34256-82-1 2.7E+02 PHYSPRO 9.1E-07 2.2E-08 PHYSPROP 2.8E-05 PHYSPROP 1.1E+01 PubChem 1.1E+00 PubChem 2.2E-02 5.6E-06 WATER9 3.0E+02 EPI 3.0E+00 PHYSPRO 2.2E+02 PHYSPROP 3.1E-02 3.4E+00 8.2E+00 5.0E-03 EPI Acetone 67-64-1 5.8E+01 PHYSPRO 1.4E-03 3.5E-05 PHYSPROP 2.3E+02 PHYSPROP -9.5E+01 PHYSPROP 7.8E-01 CRC89 1.1E-01 1.2E-05 WATER9 2.4E+00 EPI -2.4E-01 PHYSPRO 1.0E+06 PHYSPROP 1.5E-03 2.2E-01 5.3E-01 5.1E-04 EPI Acetone Cyanohydrin 75-86-5 8.5E+01 PHYSPRO 8.1E-08 2.0E-09 PHYSPROP 3.4E-01 PHYSPROP -1.9E+01 PHYSPROP 9.3E-01 CRC89 8.6E-02 1.0E-05 WATER9 1.0E+00 -

Strontium Chromate from Austria and France
Strontium Chromate from Austria and France Investigation Nos. 731-TA-1422-1423 (Preliminary) Publication 4836 October 2018 U.S. International Trade Commission Washington, DC 20436 U.S. International Trade Commission COMMISSIONERS David S. Johanson, Chairman Irving A. Williamson Meredith M. Broadbent Rhonda K. Schmidtlein Jason E. Kearns Catherine DeFilippo Director of Operations Staff assigned Kristina Lara, Investigator Samantha DeCarlo, Industry Analyst Tana von Kessler Economist Jennifer Brinckhaus, Accountant KaDeadra McNealy, Statistician David Goldfine, Attorney Douglas Corkran, Supervisory Investigator Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Strontium Chromate from Austria and France Investigation Nos. 731-TA-1422-1423 (Preliminary) Publication 4836 October 2018 CONTENTS Page ....................................................................................................................... 1 ........................................................................................................ 3 Introduction ................................................................................................................ I‐1 Background ................................................................................................................................ I‐1 Statutory criteria and organization of the report .................................................................... -

DECOMPOSITION of MIXTURES of Strontlum CHROMATE and STRONTIUM CARBONATE
PART V!. DECOMPOSITION OF MIXTURES OF STRONTlUM CHROMATE AND STRONTIUM CARBONATE. By V. T. Atlzaz~alealzd S. K. K. Jatkar. INTRBDUCTIQN. It has been shown in Part V (This Jo~trnal, 1938, ZlA, 159) that the decon~positionof strontium chromate indicated intermediate stages at 50, 66.6 and 75% clecomposition in conformity with our observations on the decomposition of calcium chromate (This Jownal, 1937, ZOA, 55-56). The stages corresponding- to 80 and 100% deconlposition could not be obtained in this case because of the lack of a suitable tube material which woulcl stand temperatures above 1450" in vacuum. While studying the decomposition of the mixtures of lime with calcium chromate (This Joz~mal, 21A, 119) two additional stages in the decomposition corresponding to 33.3 and 40% clecom- position were obtained, the composition of each stage being, respec- tively, identical with the acid-soluble portions from 66.6 and 75 % stages for plain chromate. The composition of the acid-soluble por- tion is thus an indication of the con~position of the intermediate stages obtained in the decomposition of the chromate when mixed with the corresponcling oxide. The difference in the behaviour of the con~poundsformed at the 50% stages for calcium and strontium chronlates towards acid has already been pointed out in Part V of this series. We thus get from 50% stage For strontium chromate, an acid-soluble compound 8Sr0 4Cr03 Cr,O, corresi~onclingto 33.3% decomposition, which though obtained otherwise in the decomposition of calcium chromate and its mixtures with lime, contained one mot of SrO less, ihile the corres- ponding stage in the decomposition of calcium chromate yields an acirl-soluble portion 10Ca0 6Cr0, Cr,O, A strontium compound corresponding to 33.370 stage was also obtained by treating the 66.6% stage with acid, the composition of which was 9Sr0 4Cr0, Cr,O,. -

Massachusetts Chemical Fact Sheet
Massachusetts Chemical Fact Sheet Hexavalent Chromium Table 1: HEXAVALENT CHROMIUM COMPOUNDS: Compounds SELECTED EXAMPLES* Compound Chemical Formula CAS # This fact sheet is part of a series of chemical fact sheets Ammonium chromate (NH ) Cr0 7788-98-9 developed by TURI to help Massachusetts companies, 4 2 4 community organizations and residents understand the Ammonium dichromate (NH4)2Cr2O7 7789-09-5 chemical’s use and health and environmental effects, as Barium chromate BaCrO4 10294-40-3 well as the availability of safer alternatives. tert-Butyl Chromate [(CH3)3CO]2CrO2 1189-85-1 Hexavalent chromium compounds are a toxic form of Calcium chromate CaCrO4 13765-19-0 chromium and are used in a variety of industrial processes Chromic acid H2CrO4 7738-94-5 and products. Chromium VI chloride CrCl6 14986-48-2 Hexavalent chromium compounds are human carcinogens, Chromic trioxide CrO3 1333-82-0 mutagens and developmental toxicants and are acutely Hexavalent chromium ion Cr6+ 18540-29-9 toxic. Non-hexavalent chromium compounds do not pose Lead chromate PbCrO4 7758-97-6 the same level of concern with regard to either chronic or Lead chromate oxide PbCrO4-PbO 8454-12-1 acute toxicity. Potassium chlorochromate KCrO3Cl 16037-50-6 Until 2011, all chromium compounds were treated as Potassium chromate K2CrO4 7789-00-6 a single category under TURA. Beginning with Potassium dichromate K Cr O 7778-50-9 reporting year 2012, hexavalent chromium 2 2 7 compounds are reportable under TURA as a Silver chromate Ag2CrO4 7784-01-2 separate category and are designated as a Higher Sodium chromate Na2CrO4 7775-11-3 Hazard Substance, which lowers the reporting Sodium dichromate 7789-12-0 threshold to 1,000 lb/year. -

20210311 IAEG AD-DSL V5.0 for Pdf.Xlsx
IAEGTM AD-DSL Release Version 4.1 12-30-2020 Authority: IAEG Identity: AD-DSL Version number: 4.1 Issue Date: 2020-12-30 Key Yellow shading indicates AD-DSL family group entries, which can be expanded to display a non-exhaustive list of secondary CAS numbers belonging to the family group Substance Identification Change Log IAEG Regulatory Date First Parent Group IAEG ID CAS EC Name Synonyms Revision Date ECHA ID Entry Type Criteria Added IAEG ID IAEG000001 1327-53-3 215-481-4 Diarsenic trioxide Arsenic trioxide R1;R2;D1 2015-03-17 2015-03-17 100.014.075 Substance Direct Entry IAEG000002 1303-28-2 215-116-9 Diarsenic pentaoxide Arsenic pentoxide; Arsenic oxide R1;R2;D1 2015-03-17 2015-03-17 100.013.743 Substance Direct Entry IAEG000003 15606-95-8 427-700-2 Triethyl arsenate R1;R2;D1 2015-03-17 2017-08-14 100.102.611 Substance Direct Entry IAEG000004 7778-39-4 231-901-9 Arsenic acid R1;R2;D1 2015-03-17 2015-03-17 100.029.001 Substance Direct Entry IAEG000005 3687-31-8 222-979-5 Trilead diarsenate R1;R2;D1 2015-03-17 2017-08-14 100.020.890 Substance Direct Entry IAEG000006 7778-44-1 231-904-5 Calcium arsenate R1;R2;D1 2015-03-17 2017-08-14 100.029.003 Substance Direct Entry IAEG000009 12006-15-4 234-484-1 Cadmium arsenide Tricadmium diarsenide R1;R2;D1 2017-08-14 2017-08-14 Substance Direct Entry IAEG000021 7440-41-7 231-150-7 Beryllium (Be) R2 2015-03-17 2019-01-24 Substance Direct Entry IAEG000022 1306-19-0 215-146-2 Cadmium oxide R1;R2;D1 2015-03-17 2017-08-14 100.013.770 Substance Direct Entry IAEG000023 10108-64-2 233-296-7 Cadmium -

IAEG® Declaration Development Support Document
IAEG® Declaration Development Support Document 14 July 2021 Version 2 THIS DOCUMENT IS PROVIDED BY INTERNATIONAL AEROSPACE ENVIRONMENTAL GROUP, INC. (“IAEG”) FOR INFORMATIONAL PURPOSES ONLY. ANY INACCURACY OR OMISSION IS NOT THE RESPONSIBILITY OF IAEG. IAEG DOES NOT MAKE ANY REPRESENTATIONS OR WARRANTIES WITH RESPECT TO THIS DOCUMENT OR ITS CONTENTS. IAEG HEREBY DISCLAIMS ALL WARRANTIES OF ANY NATURE, EXPRESS, IMPLIED OR OTHERWISE, OR ARISING FROM TRADE OR CUSTOM, INCLUDING, WITHOUT LIMITATION, ANY IMPLIED WARRANTIES OF MERCHANTABILITY, NONINFRINGEMENT, QUALITY, TITLE, FITNESS FOR A PARTICULAR PURPOSE, COMPLETENESS OR ACCURACY. TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAWS, IAEG SHALL NOT BE LIABLE FOR ANY LOSSES, EXPENSES OR DAMAGES OF ANY NATURE, INCLUDING, WITHOUT LIMITATION, SPECIAL, INCIDENTAL, PUNITIVE, DIRECT, INDIRECT OR CONSEQUENTIAL DAMAGES OR LOST INCOME OR PROFITS, RESULTING FROM OR ARISING OUT OF A COMPANY’S OR INDIVIDUAL’S USE OF THIS DOCUMENT, WHETHER ARISING IN TORT, CONTRACT, STATUTE, OR OTHERWISE, EVEN IF ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. IAEG IS COMMITTED TO COMPLYING WITH ALL LAWS, INCLUDING ANTITRUST LAWS. IAEG PROVIDES THIS DOCUMENT FOR INFORMATIONAL PURPOSES ONLY. DETERMINATION OF WHETHER AND/OR HOW TO USE ALL OR ANY PORTION OF THIS DOCUMENT IS TO BE MADE IN YOUR SOLE AND ABSOLUTE DISCRETION. NO PART OF THIS DOCUMENT CONSTITUTES LEGAL ADVICE OR DIRECTION. PRIOR TO USING THIS DOCUMENT, YOU SHOULD REVIEW IT, ALONG WITH APPLICABLE LAWS AND REGULATIONS, WITH YOUR OWN LEGAL COUNSEL. USE OF THIS DOCUMENT -

CM300-011 Barium Chromate
Version: 1.0 Revision date: Safety Data Sheet 09/28/2015 Supersedes: 03/01/2013 Barium Chromate 1. PRODUCT AND COMPANY IDENTIFICATION 1.1. Product Identifiers Product Form: crystalline Substance Name: Barium Chromate CAS No.: 10294-40-3 Product Code: UIC, Inc. Catalog Number CM300-011 1.2. Intended Use of the Product Use of the substance/mixture: Laboratory chemicals, Manufacture of substances, Combustion tubes Name, Address, and Telephone of the Responsible Party UIC Inc 1225 Channahon Rd Joliet, IL 60436 Phone: (815) 744-4477 Fax: (815) 744-1561 Emergency Telephone Number For Chemical Emergency, Spill, Leak, Fire, Exposure, or Accident, call emergency number: 1-815-474-8753 2. Hazards Identification of the product 2.1. Classification of the substance or mixture Oxidizing solids (Category 2), H272 Acute toxicity, Oral (Category 4), H302 Acute toxicity, Inhalation (Category 4), H332 Carcinogenicity (Category 1A), H350 For the full text of the H-Statements mentioned in this Section, see Section 16. 2.2. GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H272 May intensify fire; oxidiser. H302 + H332 Harmful if swallowed or if inhaled H350 May cause cancer. Precautionary statement(s) P201 Obtain special instructions before use. P202 Do not handle until all safety precautions have been read and understood. P210 Keep away from heat. P220 Keep/Store away from clothing/ combustible materials. P221 Take any precaution to avoid mixing with combustibles. P261 Avoid breathing dust/ fume/ gas/ mist/ vapors/ spray. P264 Wash skin thoroughly after handling. P270 Do not eat, drink or smoke when using this product. -

Exploring Equilibria Name: Investigation 1: Chromate
Exploring Equilibria Name: ________________ Chem 112 This experiment explores a variety of equilibrium systems. A reference Table of Reactions is attached to aid in your explanations. In this qualitative lab, your observations, predictions, and explanations should be recorded in the spaces provided. Using what you know about equilibria and the Table of Reactions included, you should be able to explain all your observations in terms of the relative sizes of equilibrium constants and LeChatelier’s principle. Because your investigation is qualitative, the exact amounts of the solutions you work with are not critical. You will be transferring around 1 mL of solution, which is about the same as 20 drops. Don’t use much more than that of any solution. Notice and make use of the table of some common reaction found on the last page of the handout. Investigation 1: Chromate‐Dichromate Solutions 2‐ 2‐ There are two forms of chromium in high oxidation states: chromate ion (CrO4 ) and dichromate ion (Cr2O7 ). This experiment explores the equilibrium between them. What to do: 1. Place about 0.5 mL of 1 M potassium chromate solution in a small test tube. Describe the solution. Write a balanced equation for the dissolution of K2CrO4. 2. Add a few drops of 3 M sulfuric acid (H2SO4). Write your observations. 2‐ 2‐ 3. The chromate anion (CrO4 ) is converted into dichromate ion (Cr2O7 ) in the presence of acid. Write a net ionic equation for this reaction. 4. Add several drops of 6 M sodium hydroxide (NaOH). Record your observations and write a net ionic equation for what occurs. -

Analysis of Alternatives
ANALYSIS OF ALTERNATIVES Legal name of applicants: AKZO Nobel Car Refinishes B.V. Habich GmbH; Henkel Global Supply Chain B.V.; Indestructible Paint Ltd.; Finalin GmbH; Mapaero; PPG Central (UK) Ltd in its legal capacity as Only Representative of PRC DeSoto International Inc. - OR5; PPG Industries (UK) Ltd; PPG Coatings SA; Aviall Services Inc. Submitted by: AKZO Nobel Car Refinishes B.V. Substance: Strontium chromate, EC No: 232-142-6, CAS No: 7789-06-2 Use title: Application of paints, primers and specialty coatings containing Strontium chromate in the construction of aerospace and aeronautical parts, including aeroplanes / helicopters, space craft, satellites, launchers, engines, and for the maintenance of such constructions, as well as for such aerospace and aeronautical parts, used elsewhere, where the supply chain and exposure scenarios are identical. Use number: 2 Use number: 2 Copy right protected - Property of Members of the CCST Consortium - No copying / use allowed. ANALYSIS OF ALTERNATIVES Disclaimer This document shall not be construed as expressly or implicitly granting a license or any rights to use related to any content or information contained therein. In no event shall applicant be liable in this respect for any damage arising out or in connection with access, use of any content or information contained therein despite the lack of approval to do so. ii Use number: 2 Copy right protected - Property of Members of the CCST Consortium - No copying / use allowed. ANALYSIS OF ALTERNATIVES CONTENTS 1. SUMMARY 1 2. INTRODUCTION 8 2.1. Substance 8 2.2. Uses of Cr(VI) containing substances 8 2.3. Purpose and benefits of Cr(VI) compounds 8 3. -

Barium, Zinc and Strontium Yellows in Late 19Th–Early 20Th Century Oil Paintings Vanessa Otero1* , Marta F
Otero et al. Herit Sci (2017) 5:46 DOI 10.1186/s40494-017-0160-3 RESEARCH ARTICLE Open Access Barium, zinc and strontium yellows in late 19th–early 20th century oil paintings Vanessa Otero1* , Marta F. Campos1, Joana V. Pinto2, Márcia Vilarigues1, Leslie Carlyle1 and Maria João Melo1 Abstract This work focuses on the study of the 19th century yellow chromate pigments based on barium (BaCrO 4), zinc (4ZnCrO4 K2O 3H2O) and strontium (SrCrO4). These pigments, which are reported to shift in hue and darken, have been found· in· 19th century artworks. A better understanding of their historic manufacture will contribute to the visual/chemical interpretation of change in these colours. Research was carried out on the Winsor & Newton (W&N) 19th century archive database providing a unique insight into their manufacturing processes. One hundred and three production records were found, 69% for barium, 25% for zinc and 6% for strontium chromates, mainly under the names Lemon, Citron and Strontian Yellow, respectively. Analysis of the records shows that each pigment is charac- terised by only one synthetic pathway. The low number of records found for the production of strontium chromate suggests W&N was not selling this pigment formulation on a large scale. Furthermore, contrary to what the authors have discovered for W&N chrome yellow pigments, extenders were not added to these pigment formulations, most probably due to their lower tinting strength (TS). The latter was calculated in comparison to pure chrome yellow (PbCrO4, 100% TS) resulting in 92% for barium, 65% for zinc potassium and 78% for strontium chromate pigments.