Cannabinoids and Cytochrome P450 Interactions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Analysis of Drugs Manual September 2019
Drug Enforcement Administration Office of Forensic Sciences Analysis of Drugs Manual September 2019 Date Posted: 10/23/2019 Analysis of Drugs Manual Revision: 4 Issue Date: September 5, 2019 Effective Date: September 9, 2019 Approved By: Nelson A. Santos Table of Contents CHAPTER 1 – QUALITY ASSURANCE ......................................................................... 3 CHAPTER 2 – EVIDENCE ANALYSIS ......................................................................... 93 CHAPTER 3 – FIELD ASSISTANCE .......................................................................... 165 CHAPTER 4 – FINGERPRINT AND SPECIAL PROGRAMS ..................................... 179 Appendix 1A – Definitions ........................................................................................... 202 Appendix 1B – Acronyms and Abbreviations .............................................................. 211 Appendix 1C – Instrument Maintenance Schedule ..................................................... 218 Appendix 1D – Color Test Reagent Preparation and Procedures ............................... 224 Appendix 1E – Crystal and Precipitate Test Reagent Preparation and Procedures .... 241 Appendix 1F – Thin Layer Chromatography................................................................ 250 Appendix 1G – Qualitative Method Modifications ........................................................ 254 Appendix 1H – Analytical Supplies and Services ........................................................ 256 Appendix 2A – Random Sampling Procedures -

Targeting the Endocannabinoid System to Reduce Nociception
Virginia Commonwealth University VCU Scholars Compass Theses and Dissertations Graduate School 2011 Targeting the endocannabinoid system to reduce nociception Lamont Booker Virginia Commonwealth University Follow this and additional works at: https://scholarscompass.vcu.edu/etd Part of the Medical Pharmacology Commons © The Author Downloaded from https://scholarscompass.vcu.edu/etd/2419 This Dissertation is brought to you for free and open access by the Graduate School at VCU Scholars Compass. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of VCU Scholars Compass. For more information, please contact [email protected]. Targeting the Endocannabinoid System to Reduce Nociception A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy at Virginia Commonwealth University. By Lamont Booker Bachelor’s of Science, Fayetteville State University 2003 Master’s of Toxicology, North Carolina State University 2005 Director: Dr. Aron H. Lichtman, Professor, Pharmacology & Toxicology Virginia Commonwealth University Richmond, Virginia April 2011 Acknowledgements The author wishes to thank several people. I like to thank my advisor Dr. Aron Lichtman for taking a chance and allowing me to work under his guidance. He has been a great influence not only with project and research direction, but as an excellent example of what a mentor should be (always willing to listen, understanding the needs of each student/technician, and willing to provide a hand when available). Additionally, I like to thank all of my committee members (Drs. Galya Abdrakmanova, Francine Cabral, Sandra Welch, Mike Grotewiel) for your patience and willingness to participate as a member. Our term together has truly been memorable! I owe a special thanks to Sheryol Cox, and Dr. -

Cannabinoids in Dermatology: a Scoping Review
Volume 24 Number 6| June 2018| Dermatology Online Journal || Review 24(6): 1 Cannabinoids in dermatology: a scoping review Lauren R M Eagelston1 MD, Nazanin Kuseh Kalani Yazd1 BS, Ravi R Patel2 MD, Hania K Flaten1 BS, Cory A Dunnick3,4 MD, Robert P Dellavalle3,4 MD PhD MSPH Affiliations: 1University of Colorado School of Medicine, Aurora, Colorado, USA, 2Gwinnett Medical Center, Lawrenceville, Georgia, USA, 3University of Colorado School of Medicine, Department of Dermatology, Aurora, Colorado, 4Denver Veterans Affairs Medical Center (VAMC), Department of Dermatology, Denver, Colorado Corresponding Author: Robert P. Dellavalle MD PhD MSPH, Professor of Dermatology, Chief of Dermatology Service, Department of Veteran Affairs Medical Center, 1055 Clermont Rm 6A-105 Mail Stop 16, Denver, CO 80220, Tel: 303.339.8020 x2475, Email: [email protected] Introduction Abstract The cannabinoids are a diverse class of The therapeutic applications of cannabis and pharmacologically active compounds that are cannabinoids are an increasingly conspicuous topic structurally and biologically similar to the primary as de-criminalization and legalization of these products continues to expand. A limited number of psychoactive compound derived from Cannabis cannabinoid compounds have been approved for a sativa -tetrahydrocannabinol (THC), [1-4]. There specific set of conditions. However, the current role are three classes of cannabinoids. Endogenous of cannabinoids for the treatment of dermatologic cannabinoids (endocannabinoids) occur conditions remains to be defined. We conducted a naturally/are constitutively produced in the bodies review of the current literature to determine the of humans and animals [3, 5, 6]. The most well-known applications of cannabinoids for the therapy of members of this class include 2-arachidonoyl- various skin diseases. -

Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid
Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy 231 _____________________________ _____________________________ Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid Cloning, Expression and Catalytic Properties of CYP4F8 and CYP4F21 BY JOHAN BYLUND ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2000 Dissertation for the Degree of Doctor of Philosophy (Faculty of Pharmacy) in Pharmaceutical Pharmacology presented at Uppsala University in 2000 ABSTRACT Bylund, J. 2000. Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid: Cloning, Expression and Catalytic Properties of CYP4F8 and CYP4F21. Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from Faculty of Pharmacy 231 50 pp. Uppsala. ISBN 91-554-4784-8. Cytochrome P450 (P450 or CYP) is an enzyme system involved in the oxygenation of a wide range of endogenous compounds as well as foreign chemicals and drugs. This thesis describes investigations of P450-catalyzed oxygenation of prostaglandins, linoleic and arachidonic acids. The formation of bisallylic hydroxy metabolites of linoleic and arachidonic acids was studied with human recombinant P450s and with human liver microsomes. Several P450 enzymes catalyzed the formation of bisallylic hydroxy metabolites. Inhibition studies and stereochemical analysis of metabolites suggest that the enzyme CYP1A2 may contribute to the biosynthesis of bisallylic hydroxy fatty acid metabolites in adult human liver microsomes. 19R-Hydroxy-PGE and 20-hydroxy-PGE are major components of human and ovine semen, respectively. They are formed in the seminal vesicles, but the mechanism of their biosynthesis is unknown. Reverse transcription-polymerase chain reaction using degenerate primers for mammalian CYP4 family genes, revealed expression of two novel P450 genes in human and ovine seminal vesicles. -

Transcriptomic Characterization of Fibrolamellar Hepatocellular
Transcriptomic characterization of fibrolamellar PNAS PLUS hepatocellular carcinoma Elana P. Simona, Catherine A. Freijeb, Benjamin A. Farbera,c, Gadi Lalazara, David G. Darcya,c, Joshua N. Honeymana,c, Rachel Chiaroni-Clarkea, Brian D. Dilld, Henrik Molinad, Umesh K. Bhanote, Michael P. La Quagliac, Brad R. Rosenbergb,f, and Sanford M. Simona,1 aLaboratory of Cellular Biophysics, The Rockefeller University, New York, NY 10065; bPresidential Fellows Laboratory, The Rockefeller University, New York, NY 10065; cDivision of Pediatric Surgery, Department of Surgery, Memorial Sloan-Kettering Cancer Center, New York, NY 10065; dProteomics Resource Center, The Rockefeller University, New York, NY 10065; ePathology Core Facility, Memorial Sloan-Kettering Cancer Center, New York, NY 10065; and fJohn C. Whitehead Presidential Fellows Program, The Rockefeller University, New York, NY 10065 Edited by Susan S. Taylor, University of California, San Diego, La Jolla, CA, and approved September 22, 2015 (received for review December 29, 2014) Fibrolamellar hepatocellular carcinoma (FLHCC) tumors all carry a exon of DNAJB1 and all but the first exon of PRKACA. This deletion of ∼400 kb in chromosome 19, resulting in a fusion of the produced a chimeric RNA transcript and a translated chimeric genes for the heat shock protein, DNAJ (Hsp40) homolog, subfam- protein that retains the full catalytic activity of wild-type PKA. ily B, member 1, DNAJB1, and the catalytic subunit of protein ki- This chimeric protein was found in 15 of 15 FLHCC patients nase A, PRKACA. The resulting chimeric transcript produces a (21) in the absence of any other recurrent mutations in the DNA fusion protein that retains kinase activity. -

Cannabinoids in the Pathophysiology of Skin Inflammation
molecules Review Cannabinoids in the Pathophysiology of Skin Inflammation Cristian Scheau 1 , Ioana Anca Badarau 1, Livia-Gratiela Mihai 1, Andreea-Elena Scheau 2, Daniel Octavian Costache 3, Carolina Constantin 4,5, Daniela Calina 6 , Constantin Caruntu 1,7,*, Raluca Simona Costache 8,* and Ana Caruntu 9,10 1 Department of Physiology, “Carol Davila” University of Medicine and Pharmacy, 050474 Bucharest, Romania; [email protected] (C.S.); [email protected] (I.A.B.); [email protected] (L.-G.M.) 2 Department of Radiology and Medical Imaging, Fundeni Clinical Institute, 022328 Bucharest, Romania; [email protected] 3 Department of Dermatology, “Carol Davila” Central Military Emergency Hospital, 010825 Bucharest, Romania; [email protected] 4 Immunology Department, ”Victor Babes” National Institute of Pathology, 050096 Bucharest, Romania; [email protected] 5 Department of Pathology, Colentina University Hospital, 020125 Bucharest, Romania 6 Department of Clinical Pharmacy, University of Medicine and Pharmacy of Craiova, 200349 Craiova, Romania; [email protected] 7 Department of Dermatology, “Prof. N. Paulescu” National Institute of Diabetes, Nutrition and Metabolic Diseases, 011233 Bucharest, Romania 8 Gastroenterology and Internal Medicine Clinic, Carol Davila University Central Emergency Military Hospital, Carol Davila University of Medicine and Pharmacy, 050474 Bucharest, Romania 9 Department of Oral and Maxillofacial Surgery, “Carol Davila” Central Military Emergency Hospital, 010825 Bucharest, Romania; [email protected] 10 Faculty of Medicine, “Titu Maiorescu” University, 031593 Bucharest, Romania * Correspondence: [email protected] (C.C.); [email protected] (R.S.C.); Tel.: +40-745-086-978 (C.C.) Academic Editor: Eric J. Downer Received: 30 December 2019; Accepted: 2 February 2020; Published: 4 February 2020 Abstract: Cannabinoids are increasingly-used substances in the treatment of chronic pain, some neuropsychiatric disorders and more recently, skin disorders with an inflammatory component. -

Synonymous Single Nucleotide Polymorphisms in Human Cytochrome
DMD Fast Forward. Published on February 9, 2009 as doi:10.1124/dmd.108.026047 DMD #26047 TITLE PAGE: A BIOINFORMATICS APPROACH FOR THE PHENOTYPE PREDICTION OF NON- SYNONYMOUS SINGLE NUCLEOTIDE POLYMORPHISMS IN HUMAN CYTOCHROME P450S LIN-LIN WANG, YONG LI, SHU-FENG ZHOU Department of Nutrition and Food Hygiene, School of Public Health, Peking University, Beijing 100191, P. R. China (LL Wang & Y Li) Discipline of Chinese Medicine, School of Health Sciences, RMIT University, Bundoora, Victoria 3083, Australia (LL Wang & SF Zhou). 1 Copyright 2009 by the American Society for Pharmacology and Experimental Therapeutics. DMD #26047 RUNNING TITLE PAGE: a) Running title: Prediction of phenotype of human CYPs. b) Author for correspondence: A/Prof. Shu-Feng Zhou, MD, PhD Discipline of Chinese Medicine, School of Health Sciences, RMIT University, WHO Collaborating Center for Traditional Medicine, Bundoora, Victoria 3083, Australia. Tel: + 61 3 9925 7794; fax: +61 3 9925 7178. Email: [email protected] c) Number of text pages: 21 Number of tables: 10 Number of figures: 2 Number of references: 40 Number of words in Abstract: 249 Number of words in Introduction: 749 Number of words in Discussion: 1459 d) Non-standard abbreviations: CYP, cytochrome P450; nsSNP, non-synonymous single nucleotide polymorphism. 2 DMD #26047 ABSTRACT Non-synonymous single nucleotide polymorphisms (nsSNPs) in coding regions that can lead to amino acid changes may cause alteration of protein function and account for susceptivity to disease. Identification of deleterious nsSNPs from tolerant nsSNPs is important for characterizing the genetic basis of human disease, assessing individual susceptibility to disease, understanding the pathogenesis of disease, identifying molecular targets for drug treatment and conducting individualized pharmacotherapy. -

Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma
cells Article Cannabigerol Is a Potential Therapeutic Agent in a Novel Combined Therapy for Glioblastoma Tamara T. Lah 1,2,3,*, Metka Novak 1, Milagros A. Pena Almidon 4, Oliviero Marinelli 4 , Barbara Žvar Baškoviˇc 1, Bernarda Majc 1,3, Mateja Mlinar 1, Roman Bošnjak 5, Barbara Breznik 1 , Roby Zomer 6 and Massimo Nabissi 4 1 Department of Genetic Toxicology and Cancer Biology, National Institute of Biology, 1000 Ljubljana, Slovenia; [email protected] (M.N.); [email protected] (B.Ž.B.); [email protected] (B.M.); [email protected] (M.M.); [email protected] (B.B.) 2 Faculty of Chemistry and Chemical Technology, University of Ljubljana, 1000 Ljubljana, Slovenia 3 Jožef Stefan International Postgraduate School, 1000 Ljubljana, Slovenia 4 School of Pharmacy, Experimental Medicine Section, University of Camerino, 62032 Camerino, Italy; [email protected] (M.A.P.A.); [email protected] (O.M.); [email protected] (M.N.) 5 Department of Neurosurgery, University Medical Centre Ljubljana, 1000 Ljubljana, Slovenia; [email protected] 6 MGC Pharmaceuticals d.o.o., 1000 Ljubljana, Slovenia; [email protected] * Correspondence: [email protected]; Tel.: +386-41-651-629 Simple Summary: Among primary brain tumours, glioblastoma is the most aggressive. As early relapses are unavoidable despite standard-of-care treatment, the cannabinoids delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD) alone or in combination have been suggested as a combined treatment strategy for glioblastomas. However, the known psychoactive effects of THC hamper its medical applications in these patients with potential cognitive impairment due to the progression of the Citation: Lah, T.T.; Novak, M.; Pena Almidon, M.A.; Marinelli, O.; disease. -

Cannabinoid As Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity
Cannabinoid as Potential Aromatase Inhibitor Through Molecular Modeling and Screening for Anti-Cancer Activity Sudipta Baroi1, Achintya Saha2, Ritesh Bachar3 and Sitesh C Bachar4 1Department of Pharmaceutical Technology, Faculty of Pharmacy, University of Dhaka Dhaka-1000, Bangladesh 2Department of Chemical Technology, Pharmaceutical & Fine Chemical Technology Division University of Calcutta, India 3Department of Pharmacy, School of Science and Engineering, University of Information Technology and Sciences (UITS), Dhaka-1212, Bangladesh 4Department of Pharmacy, Faculty of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh (Received: April 15, 2020; Accepted: June 2, 2020; Published (web): June 28, 2020) ABSTRACT: Inhibition of aromatase (CYTP450), a key enzyme in the estrogen biosynthesis, could result in regression of estrogen-dependent tumors and even prevent the promotion of breast cancer. The present research has been designed for searching a potent chemical moiety from natural sources to inhibit aromatase enzyme, the over- functionality of which causes the breast cancer. Cannabis sativa contains a very much promising group of cannabinoids with more than 66 compounds with reported anticancer property and for the search of a target specific potent aromatase inhibitor, 61 cannabinoids from C. sativa were selected. The Structures Data File (SDF) of these ligand molecules were subjected to docking studies at the binding site of aromatase X-ray crystallographic structure based on lower resolution of the protein crystal structure and higher docking accuracy, predicted by calculating the correlation between experimental activities and Glide dock scores and compared with the standard aromatase ligand androstenedione and aromatase inhibitor fadrozole with existing drug for breast cancer treatment. The best docked pose of each ligand was selected on the basis of the highest dock score related to the binding free energies of the internal dataset compounds as compared to their observed activities. -
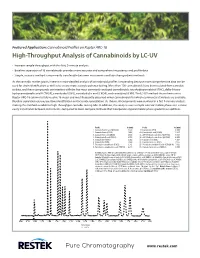
High-Throughput Analysis of Cannabinoids by LC-UV
Featured Application: Cannabinoid Profiles on Raptor ARC-18 High-Throughput Analysis of Cannabinoids by LC-UV • Increase sample throughput with this fast, 9-minute analysis. • Baseline separation of 16 cannabinoids provides more accurate and comprehensive potency and profile data. • Simple, isocratic method is more easily transferable between instruments and labs than gradient methods. As the cannabis market grows, interest in more detailed analysis of cannabinoid profiles is expanding because more comprehensive data can be used for strain identification as well as to ensure more accurate potency testing. More than 100 cannabinoids have been isolated from cannabis to date, and these compounds can interfere with the five most commonly analyzed cannabinoids: tetrahydrocannabinol (THC), delta-9-tetra- hydrocannabinolic acid A (THCA), cannabidiol (CBD), cannabidiolic acid (CBDA), and cannabinol (CBN). The LC-UV method shown here uses a Raptor ARC-18 column to fully resolve 16 major and most frequently observed minor cannabinoids for which commercial standards are available. Baseline separation ensures positive identification and accurate quantitation. As shown, all compounds were resolved in a fast 9-minute analysis, making this method suitable for high-throughput cannabis testing labs. In addition, this analysis uses a simple isocratic mobile phase so it is more easily transferable between instruments, compared to more complex methods that incorporate atypical mobile phase gradients or additives. Peaks tR (min) Peaks tR (min) 1. Cannabidivarinic acid (CBDVA) 1.877 9. Cannabinol (CBN) 4.609 2. Cannabidivarin (CBDV) 2.086 10. Cannabinolic acid (CBNA) 5.437 3. Cannabidiolic acid (CBDA) 2.592 11. Δ9-Tetrahydrocannabinol (Δ9-THC) 5.815 4. Cannabigerolic acid (CBGA) 2.750 12. -

CBD (Cannabidiol)
TRANSPORTATION RESEARCH BOARD Driving Toward the Truth - Dispelling the Myths About Cannabis Products February 10, 2021 @NASEMTRB #TRBwebinar PDH Certification The Transportation Research Board has met the standards and Information: requirements of the Registered Continuing Education Providers •1.5 Professional Development Program. Credit earned on completion Hour (PDH) – see follow-up of this program will be reported to email for instructions RCEP. A certificate of completion will •You must attend the entire be issued to participants that have registered and attended the entire webinar to be eligible to receive session. As such, it does not include PDH credits content that may be deemed or •Questions? Contact Reggie construed to be an approval or Gillum at [email protected] endorsement by RCEP. #TRBwebinar Learning Objectives 1. Identify impacts of the Farm Bill on use of THC and CBD products 2. Describe the toxicology of THC and CBD products 3. Discuss how THC and CBD products affect driving performance and crash risk #TRBwebinar TRB Standing Committee on Impairment in Transportation (ACS50) TRB Webinar: Driving Toward the Truth - Dispelling the Myths About Cannabis Products Dr. Barry K. Logan Executive Director, Center for Forensic Science Research and Education (CFSRE); Senior Vice President of Forensic Sciences, and Chief Scientist at NMS Labs Michelle Peace, Ph.D. Associate Professor and PI, Laboratory for Forensic Toxicology Research Department of Forensic Science, Virginia Commonwealth University Dr. Darrin Grondel Vice President, -

Potential Health Benefits of Cannabis Extracts: a Review Maria Rosana Ramirez
International Journal of Chemical and Biomedical Science 2016; 2(1): 1-8 Published online March 1, 2016 (http://www.aascit.org/journal/ijcbs) Potential Health Benefits of Cannabis Extracts: A Review Maria Rosana Ramirez CITER-The National Scientific and Technical Research Council (CONICET), Argentine Government, Agency, Argentina Email address [email protected] Citation Maria Rosana Ramirez. Potential Health Benefits of Cannabis Extracts: A Review. International Journal of Chemical and Biomedical Science . Vol. 2, No. 1, 2016, pp. 1-8. Keywords Abstract Non-cannabinoids Compounds, A central tenet underlying the use of plant preparations is that herbs contain many Cannabis Extracts, bioactive compounds. Cannabis contains tetrahydrocannabinols (THC) a primary Bioactivity metabolite with reported psychotropic effects. Therefore, the presence of THC makes controversial the use of Cannabis to treat diseases by which their uses and applications were limited. The question then is: is it possible to use the extracts from Cannabis to treat the diseases related with it use in folk medicine? More recently, the synergistic Received: January 24, 2016 contributions of bioactive constituents have been scientifically demonstrated. We Revised: February 10, 2016 reviewed the literature concerning medical cannabis and its secondary metabolites, Accepted: February 12, 2016 including fraction and total extracts. Scientific evidence shows that secondary metabolites in cannabis may enhance the positive effects of THC a primary metabolite. Other chemical components (cannabinoid and non-cannabinoid) in cannabis or its extracts may reduce THC-induced anxiety, cholinergic deficits, and immunosuppression; which could increase its therapeutic potential. Particular attention will be placed on non- cannabinoid compounds interactions that could produce synergy with respect to treatment of pain, inflammation, epilepsy, fungal and bacterial infections.