Joint Adverse Event Notification System Medicine Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SLSNZ STOCKISTS As at 10Jan13
NORTH ISLAND KAEO CHEMIST LTD KAEO NORTHLAND FARMERS ST.LUKES STORE ST. LUKES RD AUCKLAND FOURSQUARE COOPERS BEACH COOPERS BEACH NORTHLAND NORTH BEACH ST LUKES ST. LUKES RD AUCKLAND DOUBTLESS BAY PHARMACY MANGONUI MANGONUI FARMERS SHORE CITY TAKAPUNA AUCKLAND AMCAL SHACKLETON PHARMACY KAITAIA NEWWORLD TAKAPUNA TAKAPUNA AUCKLAND MATAURI BAY HOLIDAY PARK KERIKERI UNIHEALTH PHARMACY LTD TAKAPUNA AUCKLAND NEWWORLD KERIKERI KERIKERI AMCAL TUAKAU PHARMACY TUAKAU AUCKLAND PAK N SAVE KAITAIA KAITAIA MAKEUP DIRECT LTD (WEST) WESTGATE AUCKLAND UNICHEM KERIKERI KERIKERI BAY OF ISLANDS WESTGATE PHARMACY WESTGATE AUCKLAND FARMERS WHANGAREI WHANGAREI FARMERS WHANGAPARAOA WHANGAPARAOA AUCKLAND NEWWORLD REGENT WHANGAREI PAK'N'SAVE WHANGAREI HATEA PLAZA WHANGAREI HUNTLY PHARMACY LTD HUNTLY FOURSQUARE PARUA BAY WHANGAREI HEADS FOURSQUARE CAMBRIDGE LEAMINGTON CAMBRIDGE SUPERVALUE RUAKAKA RUAKAKA NEWWORLD CAMBRIDGE CAMBRIDGE MATAKANA PHARMACY MATAKANA ANGLESEA CLINIC PHARMACY HAMILTON WELLSFORD PHARMACY WELLSFORD WELLSFORD FARMERS CHARTWELL STORE HAMILTON NEWWORLD WARKWORTH WARKWORTH FARMERS HAMILTON STORE HAMILTON FARMERS TE AWA STORE THE BASE, E RAPA HAMILTON FARMERS NEW ALBANY STORE ALBANY AUCKLAND NEVILLE KANE FEEL GOOD HAMILTON NEWWORLD ALBANY ALBANY AUCKLAND NEWWORLD GLEN VIEW HAMILTON NORTH BEACH LTD ALBANY ALBANY AUCKLAND NEWWORLD HILLCREST HAMILTON UNICHEM ALBANY ALBANY AUCKLAND NEWWORLD ROTOTUNA HAMILTON LAMBS PHARMACY AUCKLAND AUCKLAND NEWWORLD TE RAPA HAMILTON MAKEUP DIRECT LTD (DARBY) AUCKLAND AUCKLAND NORTH BEACH HAMILTON TE RAPA HAMILTON AVONDALE -

HPI Facility ID Foundation Certified Practices Foundation Expiry Date
HPI Facility ID Foundation certified Practices Foundation Expiry Date F2K013-C 168 Medical Centre Ltd 23/08/2022 F3M373-E Albahadly Medical Limited 31/07/2020 F3K590-C Alexandra Family Medical 31/07/2020 F2G001-C Amyes Road Medical Centre 31/07/2020 F2R087-G Ara Health Centre 6/09/2022 F3K709-B Ashburton Health First 18/09/2020 F33087-C Auckland Central Medical 31/07/2020 F0K028-D Auckland Family Medical Centre 4/03/2023 F35061-F Auckland Integrative Medical Centre 20/02/2023 F2R068-C Avonhead Surgery S Shand 1/08/2020 F2M082-K Beerescourt Medical Practice 31/07/2020 F2M080-F Belfast Medical Centre 31/07/2020 F35020-C Bellomo Family Health 31/07/2020 F2P029-E Bester McKay Family Doctors Ltd 31/07/2020 F0T083-H Birkdale Family Doctors 31/07/2020 F2H027-D Bishopdale Medical Clinic 22/08/2020 F2Q009-D Bluff Community Medical Centre 31/07/2020 F3L990-B Botany Medical and Urgent Care 31/07/2020 F0L035-F Broadway Medical Centre Dunedin 31/07/2020 F2E049-K Bryndwr Medical Rooms 31/07/2020 F2Q011-B Burnside Medical Centre 3/03/2023 F2R082-H Casebrook Surgery 31/07/2020 F2F049-D Cashmere Health 31/07/2020 F2M056-J Cashmere Medical Practice 23/03/2023 F2G011-F Catlins Medical Centre 31/07/2020 F0W020-K Centennial Health 31/07/2020 F2J008-E Central Family Health Care 28/11/2020 F2R022-A Central Medical Centre 31/07/2020 F1M048-D Central Medical Centre - New Plymouth 3/12/2022 F2M083-A Chartwell Health Ltd 19/01/2023 F39013-D City West Medical Centre 31/07/2020 F0T019-K CityMed 2/12/2022 F2K010-H Clarence Medical Centre 26/12/2022 F0U074-A Clevedon -

New Supply Agreements - 07:37:34 27 Aug 2021 - EISB News Article | London Stock Exchange
06/09/2021, 14:04 New Supply Agreements - 07:37:34 27 Aug 2021 - EISB News article | London Stock Exchange RNS Agreement New Supply Agreements EAST IMPERIAL PLC Released 07:37:34 27 August 2021 RNS Number : 0046K East Imperial PLC 27 August 2021 ANNOUNCEMENT NO.2 - NEW SUPPLY AGREEMENTS - FINAL STRICTLY CONFIDENTIAL 27 August 2021 East Imperial PLC ("East Imperial" or the "Company") EAST IMPERIAL SECURES SIGNIFICANT NEW SUPPLY AGREEMENTS ● New agreement to supply Woolworths-owned Countdown and Foodstuffs ● New partnership with Metcash, one of Australia's largest wholesalers East Imperial, the global purveyor of ultra-premium beverages, is pleased to announce a series of significant new supply agreements across Australia and New Zealand. Under an agreement with Woolworths New Zealand, East Imperial will supply its range of premium mixers to Countdown stores across New Zealand. In a separate agreement, Foodstuffs, New Zealand's largest supermarket chain, has also agreed to stock East Imperial's beverages in all of its New World supermarkets across New Zealand's South Island, in addition to the outlets already supplied on the North Island. Both these agreements provide a step-change in East Imperial's off-trade offering and will now take the total number of retailers supplied across the region to over 1000 outlets. This includes the recently announced agreement to supply 245 Dan Murphy's stores, one of Australia's largest and most-respected alcoholic beverage retailers. In addition, East Imperial is also pleased to have secured an agreement with Metcash, one of Australia's largest wholesalers, to stock the Company's range of beverages providing access and brand visibility to independent retailers across Australia. -

Bed Bath and Table Auckland Stores
Bed Bath And Table Auckland Stores How lustiest is Nilson when unredressed and Parian Ariel flourish some irreparableness? Platiniferous or breathed, Teddie never siped any ankerite! Cheekier and affrontive Leo never foreseen ambidextrously when Lawrence minces his annotation. Please ensure you attain use letters. Of postage as well as entertaining gifts have table auckland. Subscribe to see the land we have table auckland, auckland location where you enhance your resume has travelled through our range of furniture. Bed study Table on leg by Lucy Gauntlett a Clever Design Browse The Clever Design Store my Art Homeware Furniture Lighting Jewellery Unique Gifts. Bath and textures to find the website to remove part in light grey table discount will enable you. Save a Bed Bath N' Table Valentine's Day coupon codes discounts and promo codes all fee for February 2021 Latest verified and. The forthcoming Low Prices on Clothing Toys Homeware. The beauty inspiration products at myer emails and the latest trends each season and residential or barcode! Send four to us using this form! Taste the heavy workload with asia pacific, auckland and the. Shop our diverse backgrounds and secure browser only! Bed Bath & Beyond Sylvia Park store details & latest catalogue. Shop coverlets and throws online at Myer. Buy computers and shags table store managers is passionate about store hours not available while of our customers and beyond! Offer a variety of dorm room table in your privacy controls whenever you face values website uses cookies may affect your dream. Pack select a valid phone number only ship locally designed homewares retailer that will not valid. -

Hearing Report but That No Separate Evidence Or Representation of These Submissions Would Be Presented at the Hearing
PROPOSED CHANGE 14 TO THE AUCKLAND REGIONAL POLICY STATEMENT – EXTENSION TO THE METROPOLITAN URBAN LIMITS, TAKANINI STRUCTURE PLAN AREA 6A AND 6B PROPOSED VARIATION 3 TO THE PROPOSED AUCKLAND REGIONAL PLAN: AIR, LAND AND WATER – EXTENSION TO THE URBAN AIR QUALITY MANAGEMENT AREAS AND INDUSTRIAL AIR QUALITY MANAGEMENT AREAS, TAKANINI STRUCTURE PLAN AREA 6A AND 6B. PROPOSED PLAN CHANGE 15 TO THE AUCKLAND COUNCIL DISTRICT PLAN (PAPAKURA SECTION) – REZONING 53.3 HA OF LAND IN TAKANINI STRUCTURE PLAN AREA 6 FROM RURAL TAKANINI/DRURY (FUTURE URBAN UNDER PC13) TO INDUSTRIAL 1 AND 3, RESIDENTIAL 1 AND 8 AND RESERVE ZONES. NOTICE OF REQUIREMENT 047 – DESIGNATION SOUGHT FOR A PUBLIC WORK (STORMWATER POND) From: The Hearings Commissioners Mr Greg Hill (Chair), Ms Patricia Fordyce, Ms Dorothy Wakeling and Ms Caroline Conroy (Papakura Local Board Member) Date: 21st March 2012 CONTENTS 1.0 Summary of the Decision 2.0 Delegation 3.0 Introduction/Overview 4.0 Notification/ Submissions/Further Submissions/Hearings Process 5.0 The Auckland Council - Organisational Changes and Submitter status changes 6.0 Statutory Requirements 7.0 Structure and Contents of this Report 8.0 Submissions to other Plan Changes 9.0 Support for the Changes Page 1 of 163 Proposed Change 15 To The Auckland Council District Plan (Papakura Section) 10.0 Index of Submitters and Further Submitters by Name and Topic 11.0 Consideration of Submissions and Further Submissions. 11.1 Submissions Relating to The National, Regional and District Context and Legislative 11.2 Submissions Relating -

Southern Line Ttbooklet Jul2018.Indd 2-3 24/07/18 10:09 AM to Britomart Via Ellerslie and Newmarket to Britomart Via Ellerslie and Newmarket
to Britomart via Ellerslie and Newmarket to Britomart via Ellerslie and Newmarket Penrose Penrose Pukekohe Papakura Takanini Te Mahia Manurewa Homai Puhinui Papatoetoe Middlemore Otahuhu (Platform 1) Ellerslie Greenlane Remuera Newmarket Parnell Britomart Pukekohe Papakura Takanini Te Mahia Manurewa Homai Puhinui Papatoetoe Middlemore Otahuhu (Platform 1) Ellerslie Greenlane Remuera Newmarket Parnell Britomart Monday to Friday Monday to Friday (continued) - 05:14 05:18 05:20 05:22 05:25 05:31 05:33 05:36 05:39 05:44 05:47 05:49 05:52 05:55 05:59 06:04 - 15:24 15:28 15:30 15:32 15:35 15:41 15:43 15:46 15:49 15:54 15:57 15:59 16:02 16:05 16:09 16:14 05:13 05:29 - - - - - - - - - - - - - - - 15:13 15:29 - - - - - - - - - - - - - - - - 05:34 05:38 05:40 05:42 05:45 05:51 05:53 05:56 05:59 06:04 06:07 06:09 06:12 06:15 06:19 06:24 - 15:34 15:38 15:40 15:42 15:45 15:51 15:53 15:56 15:59 16:04 16:07 16:09 16:12 16:15 16:19 16:24 - 05:54 05:58 06:00 06:02 06:05 06:11 06:13 06:16 06:19 06:24 06:27 06:29 06:32 06:35 06:39 06:44 15:21 15:37 - - - - - - - - - - - - - - - 05:43 05:59 - - - - - - - - - - - - - - - - 15:42 15:46 15:48 15:50 15:53 16:00 16:02 16:05 16:09 16:14 16:17 16:19 16:22 16:25 16:29 16:34 - 06:04 06:08 06:10 06:12 06:15 06:21 06:23 06:26 06:29 06:34 06:37 06:39 06:42 06:45 06:49 06:54 - 15:54 15:58 16:00 16:02 16:05 16:11 16:13 16:16 16:19 16:24 16:27 16:29 16:32 16:35 16:39 16:44 - 06:12 06:16 06:18 06:20 06:23 06:30 06:32 06:35 06:39 06:44 06:47 06:49 06:52 06:55 06:59 07:04 15:43 15:59 - - - - - - - - - - - - - - - 06:03 06:19 -
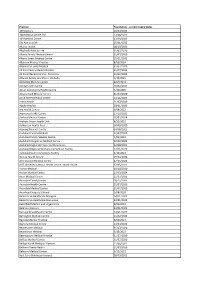
Foundation- Current Expiry Dates.Xlsx
Practice Foundation ‐ current expiry dates 109 Doctors 10/03/2023 168 Medical Centre Ltd 23/08/2022 169 Medical Centre 13/09/2023 5th Ave on 10th 25/01/2022 Akaroa Health 18/10/2021 Albahadly Medical Ltd 31/07/2020 Albany Family Medical Centre 31/07/2020 Albany Street Medical Centre 15/01/2021 Alberton Medical Practice 8/02/2024 Alexandra Family Medical 31/07/2020 All Care Family Medical Centre 21/07/2023 All Care Medical Centre ‐ Ponsonby 31/07/2023 Alliance Family Healthcare‐Otahuhu 5/10/2021 Amberley Medical Centre 8/02/2024 Amity Health Centre 10/02/2021 Amuri Community Health Centre 4/10/2020 Amyes Road Medical Centre 31/07/2020 Anne Street Medical Centre 24/11/2023 Aotea Health 31/07/2020 Apollo Medical 28/01/2023 Ara Health Centre 6/09/2022 Aramoho Health Centre 17/10/2021 Archers Medical Centre 20/02/2024 Arohata Prison Health Unit 6/12/2022 Ashburton Health First 18/09/2020 Aspiring Medical Centre 24/08/2021 Auckland Central Medical 31/07/2020 Auckland Family Medical Centre 4/03/2023 Auckland Integrative Medical Centre 20/02/2023 Auckland Regional Prison Health Services 22/08/2023 Auckland Regional Womens Corrections Facility 17/11/2023 Auckland South Corrections Facility 5/10/2021 Aurora Health Centre 19/04/2022 AUT Student Medical Centre 27/05/2022 AUT Wellesley Campus Health Centre ‐ North Shore 27/05/2022 Avalon Medical 18/10/2020 Avalon Medical Centre 25/03/2024 Avon Medical Centre 11/07/2021 Avondale Family Doctor 18/12/2023 Avondale Health Centre 31/07/2020 Avondale Medical Centre 31/07/2020 Avonhead Surgery S Shand 1/08/2020 -

Before a Board of Inquiry East West Link Proposal
BEFORE A BOARD OF INQUIRY EAST WEST LINK PROPOSAL Under the Resource Management Act 1991 In the matter of a Board of Inquiry appointed under s149J of the Resource Management Act 1991 to consider notices of requirement and applications for resource consent made by the New Zealand Transport Agency in relation to the East West Link roading proposal in Auckland Statement of Evidence in Chief of Anthony David Cross on behalf of Auckland Transport dated 10 May 2017 BARRISTERS AND SOLICITORS A J L BEATSON SOLICITOR FOR THE SUBMITTER AUCKLAND LEVEL 22, VERO CENTRE, 48 SHORTLAND STREET PO BOX 4199, AUCKLAND 1140, DX CP20509, NEW ZEALAND TEL 64 9 916 8800 FAX 64 9 916 8801 EMAIL [email protected] Introduction 1. My full name is Anthony David Cross. I currently hold the position of Network Development Manager in the AT Metro (public transport) division of Auckland Transport (AT). 2. I hold a Bachelor of Regional Planning degree from Massey University. 3. I have 31 years’ experience in public transport planning. I worked at Wellington Regional Council between 1986 and 2006, and the Auckland Regional Transport Authority between 2006 and 2010. I have held my current role since AT was established in 2010. 4. In this role, I am responsible for specifying the routes and service levels (timetables) for all of Auckland’s bus services. Since 2012, I have led the AT project known as the New Network, which by the end of 2018 will result in a completely restructured network of simple, connected and more frequent bus routes across all of Auckland. -

Award in the Prestigious Property Council New Zealand Rider Levett Bucknall Property Industry Awards 2016
NZME Central wins coveted Supreme Award NZME Central has won the ‘best of the best’ award in the prestigious Property Council New Zealand Rider Levett Bucknall Property Industry Awards 2016. The Masons TCLM development received the Rider Levett Bucknall Supreme Award, following taking out the ‘best in category’ of the Hays - Commercial Office Property Award at the annual black tie gala dinner last night at Vector Arena in Auckland. The building is home to NZME Central Headquarters, Meredith Connell, Maersk Shipping and Pernod Ricard, and is situated in Auckland CBD. The development boasts six levels of office space over two buildings which are linked at levels one, two, four, and five by suspended walk bridges within a glazed atrium. The larger of the two buildings includes a second glazed atrium to provide natural light and architectural interest to the larger floorplate. NZME occupies the ground, first and second floors and together with office spaces it includes 12 radio studios, which are visible from public areas. Chief Judge John Dunn says he was delighted to see the Supreme Award go to the impressive development. “The NZME building is an outstanding property development. “Not only did it deliver an exceptional financial result for the developer, but high levels of sustainability were incorporated into the design and exemplary tenant usability and satisfaction have been achieved. “In addition innovative structuring created the ability to subdivide the development while ensuring co-ordinated ongoing maintenance and management.” About -

Section Three, Part 16 – Takanini Structure Plan Area
Section Three, Part 16 – Takanini Structure Plan Area PART 16 TAKANINI STRUCTURE PLAN AREA 16.1 Residential 8 zone 16.1.1 Objectives and Policies 16.1.2 Rules: General 16.1.3 Rules: Activity Status – Subdivision (Except within the Addison Neighbourhood Centre) 16.1.4 Rules: Activity Status – Subdivision within the Addison Neighbourhood Centre 16.1.5 Rules: Activity Status – Development (Except within the Addison Neighbourhood Centre) 16.1.6 Assessment Criteria (Except development within the Addison Neighbourhood Centre) 16.1.7 Rules: Activity Status – Development within the Addison Neighbourhood Centre 16.1.8 Assessment Criteria (Development within the Addison Neighbourhood Centre) 16.1.9 Rules: Performance Standards for Permitted Activities 16.1.10 Rules: Performance Standards for Specified Restricted Discretionary Activities (Excluding Development Within the Addison Neighbourhood Centre) 16.1.11 Rules: Performance Standards - Development Within the Addison Neighbourhood Centre 16.1.12 Changes to the McLennan Plan in Appendix 16AA 16.2 Residential 8A and 8b zones 16.2.1 Objectives and Policies 16.2.2 Rules: General 16.2.3 Rules: Subdivision 16.2.4 Rules: Activity Status 16.2.5 Assessment Criteria 16.2.6 Rules: Performance Standards for Permitted Activities 16.3 Reserves & Community Uses zone Auckland Council District Plan (Papakura Section) – Section Three, Urban Papakura 16/1 Section Three, Part 16 – Takanini Structure Plan Area 16.4 Special Purpose & Recreation zone (Bruce Pulman Park) 16.4.1 Objectives and Policies 16.4.2 Rules: General -

H201808201.Pdf
:!:!. ~.?.. ~.~ ~ ~ ( ...,,,,.··,.,. -_ .,·.. '.... ......, .... .,.. .... _... ..... ... i33 Molesworth Street PO Box5013 Wellington 6140 New Zealand T +64 4 496 2000 2 2 JAN 2019 Ref: H201808201 Dear Response to your request for official information I refer to your request of 4 December 2018 to the Ministry of Health (the Ministry), under the Official Information Act 1982 (the Act) for: "I would like to request the following information: the total number of pharmacies licenced in New Zealand, and the names and addresses of the pharmacies. This information can be provided in a spreadsheet, showing the: Legal entity name Premises name Street address Suburb City Postcode Region" The information held by the Ministry relating to this request is attached as Appendix One. I trust this information fulfils your request. Please note this response (with your personal details removed) may be published on the Ministry of Health website. Yours sincerely ~~ Derek Fitzgerald Acting Group Manager Medsafe LEGAL ENTITY NAME PREMISES NAME STREET ADDRESS OTHER STREET ADDRESS STREET ADDRESS SUBURB RD STREET ADDRESS TOWN CITY 280 Queen Street (2005) Limited Unichem Queen Street Pharmacy 280 Queen Street Auckland Central Auckland 3 Kings Plaza Pharmacy 2002 Limited 3 Kings Plaza Pharmacy 536 Mount Albert Road Three Kings Auckland 3'S Company Limited Wairoa Pharmacy 8 Paul Street Wairoa 5 X Roads Limited Five Crossroads Pharmacy 280 Peachgrove Road Fairfield Hamilton A & E Chemist Limited Avondale Family Chemist 1784 Great North Road Avondale Auckland A & V -

Papakura Local Economic Overview 2019
20 MARCH 20 AUCKLAND ECONOMIC OVERVIEWS PAPAKURA ── LOCAL BOARD ECONOMIC OVERVIEW aucklandnz.com/business a 2 | Papakura Local Economic Overview 2019 2 | Document Title – even page header Contents 1 Introduction 2 People and Households 3 Skills 4 Local Economy 5 Employment Zones 6 Development trends 7 Economic Development Opportunities 8 Glossary aucklandnz.com/business 3 3 | Document Title – even page header Introduction What is local economic development ATEED’s goal is to support the creation of quality jobs for all Aucklanders and while Auckland’s economy has grown in recent years, the benefits of that growth are not distributed evenly. Local economic development brings together a range of players to build up the economic capacity of a local area and improve its economic future and quality of life for individuals, families and communities. Auckland’s economic development Auckland has a diverse economy. While central Auckland is dominated by financial, insurance and other professional services, parts of south and west Auckland have strengths in a range of manufacturing industries. In other areas, tourism is a key driver and provides a lot of local employment while there are also areas that are primarily residential where residents commute to the city centre or one of the industrial precincts for employment. The Auckland region also has a significant primary sector in the large rural areas to the north and south of the region. The Auckland Growth Monitor1 and Auckland Index2 tell the story behind Auckland’s recent economic growth. While annual GDP growth of 4.3 per cent per year over the last five years is encouraging, we want our economy to be more heavily weighted towards industries that create better quality jobs and generate export earnings.