Aquatic Feeding in Salamanders
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pond-Breeding Amphibian Guild
Supplemental Volume: Species of Conservation Concern SC SWAP 2015 Pond-breeding Amphibians Guild Primary Species: Flatwoods Salamander Ambystoma cingulatum Carolina Gopher Frog Rana capito capito Broad-Striped Dwarf Siren Pseudobranchus striatus striatus Tiger Salamander Ambystoma tigrinum Secondary Species: Upland Chorus Frog Pseudacris feriarum -Coastal Plain only Northern Cricket Frog Acris crepitans -Coastal Plain only Contributors (2005): Stephen Bennett and Kurt A. Buhlmann [SCDNR] Reviewed and Edited (2012): Stephen Bennett (SCDNR), Kurt A. Buhlmann (SREL), and Jeff Camper (Francis Marion University) DESCRIPTION Taxonomy and Basic Descriptions This guild contains 4 primary species: the flatwoods salamander, Carolina gopher frog, dwarf siren, and tiger salamander; and 2 secondary species: upland chorus frog and northern cricket frog. Primary species are high priority species that are directly tied to a unifying feature or habitat. Secondary species are priority species that may occur in, or be related to, the unifying feature at some time in their life. The flatwoods salamander—in particular, the frosted flatwoods salamander— and tiger salamander are members of the family Ambystomatidae, the mole salamanders. Both species are large; the tiger salamander is the largest terrestrial salamander in the eastern United States. The Photo by SC DNR flatwoods salamander can reach lengths of 9 to 12 cm (3.5 to 4.7 in.) as an adult. This species is dark, ranging from black to dark brown with silver-white reticulated markings (Conant and Collins 1991; Martof et al. 1980). The tiger salamander can reach lengths of 18 to 20 cm (7.1 to 7.9 in.) as an adult; maximum size is approximately 30 cm (11.8 in.). -
Comparative Osteology and Evolution of the Lungless Salamanders, Family Plethodontidae David B
COMPARATIVE OSTEOLOGY AND EVOLUTION OF THE LUNGLESS SALAMANDERS, FAMILY PLETHODONTIDAE DAVID B. WAKE1 ABSTRACT: Lungless salamanders of the family Plethodontidae comprise the largest and most diverse group of tailed amphibians. An evolutionary morphological approach has been employed to elucidate evolutionary rela tionships, patterns and trends within the family. Comparative osteology has been emphasized and skeletons of all twenty-three genera and three-fourths of the one hundred eighty-three species have been studied. A detailed osteological analysis includes consideration of the evolution of each element as well as the functional unit of which it is a part. Functional and developmental aspects are stressed. A new classification is suggested, based on osteological and other char acters. The subfamily Desmognathinae includes the genera Desmognathus, Leurognathus, and Phaeognathus. Members of the subfamily Plethodontinae are placed in three tribes. The tribe Hemidactyliini includes the genera Gyri nophilus, Pseudotriton, Stereochilus, Eurycea, Typhlomolge, and Hemidac tylium. The genera Plethodon, Aneides, and Ensatina comprise the tribe Pleth odontini. The highly diversified tribe Bolitoglossini includes three super genera. The supergenera Hydromantes and Batrachoseps include the nominal genera only. The supergenus Bolitoglossa includes Bolitoglossa, Oedipina, Pseudoeurycea, Chiropterotriton, Parvimolge, Lineatriton, and Thorius. Manculus is considered to be congeneric with Eurycea, and Magnadig ita is congeneric with Bolitoglossa. Two species are assigned to Typhlomolge, which is recognized as a genus distinct from Eurycea. No. new information is available concerning Haptoglossa. Recognition of a family Desmognathidae is rejected. All genera are defined and suprageneric groupings are defined and char acterized. Range maps are presented for all genera. Relationships of all genera are discussed. -

Vernacular Name AMPHIUMA, ONE-TOED (Aka: Congo Eel, Congo Snake, Ditch Eel, Fish Eel and Lamprey Eel)
1/6 Vernacular Name AMPHIUMA, ONE-TOED (aka: Congo Eel, Congo Snake, Ditch Eel, Fish Eel and Lamprey Eel) GEOGRAPHIC RANGE Eastern Gulf coast. HABITAT Wetlands: slow moving or stagnant freshwater rivers/streams/creeks and bogs, marshes, swamps, fens and peat lands. CONSERVATION STATUS IUCN: Near Threatened (2016). Population Trend: Decreasing. Because of the limited extent of its occurrence and because of the declining extent and quality of its habitat, this species is close to qualifying for Vulnerable. COOL FACTS Amphiumas are commonly known as "Congo eels," a misnomer. First of all, amphiumas are amphibians, rather than fish (which eels are). This notwithstanding, amphiumas bear resemblance to the elongate fishes. It is easy to overlook the diminutive legs, and the lack of any external gills adds to the similarity between the amphiumas and eels. Amphiumas are adapted for digging and tunneling. They seem to spend most of the time in muddy burrows and are rarely observed in the wild. They never fully metamorphose and retain larval characteristics in varying degrees into adulthood: one pair of the larval gill slits is retained and never disappears, no eyelids, no tongue and the presence of 4 gill arches with a single respiratory opening between the 3 rd--4th arches. Amphiumas have two pairs of limbs, and the three species, all of which occur in the S.E. U.S, differ in regard to the number of toes at the ends of these limbs: one, two or three. These amphiumas possess tiny, single-toed limbs, one pair just behind the small gill opening at each side of the neck and another pair just ahead of the longitudinal anal slit . -

Reproductive Biology of the Southern Dwarf Siren, Pseudobranchus Axanthus, in Southern Florida Zachary Cole Adcock University of South Florida, [email protected]
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School January 2012 Reproductive Biology of the Southern Dwarf Siren, Pseudobranchus axanthus, in Southern Florida Zachary Cole Adcock University of South Florida, [email protected] Follow this and additional works at: http://scholarcommons.usf.edu/etd Part of the American Studies Commons, and the Biology Commons Scholar Commons Citation Adcock, Zachary Cole, "Reproductive Biology of the Southern Dwarf Siren, Pseudobranchus axanthus, in Southern Florida" (2012). Graduate Theses and Dissertations. http://scholarcommons.usf.edu/etd/3941 This Thesis is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Reproductive Biology of the Southern Dwarf Siren, Pseudobranchus axanthus, in Southern Florida by Zachary C. Adcock A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science Department of Integrative Biology College of Arts and Sciences University of South Florida Co-Major Professor: Earl D. McCoy, Ph.D. Co-Major Professor: Henry R. Mushinsky, Ph.D. Stephen M. Deban, Ph.D. Date of Approval: July 10, 2012 Keywords: aquatic salamander, life history, oviposition, clutch size, size at maturity Copyright © 2012, Zachary C. Adcock ACKNOWLEDGMENTS I thank my co-major professors, Henry Mushinsky and Earl McCoy, for their patience and guidance through a couple of thesis projects lasting several years. I also thank my committee member, Steve Deban, for providing excellent comments to improve this thesis document. -

TRAPPING SUCCESS and POPULATION ANALYSIS of Siren Lacertina and Amphiuma Means
TRAPPING SUCCESS AND POPULATION ANALYSIS OF Siren lacertina AND Amphiuma means By KRISTINA SORENSEN A THESIS PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE UNIVERSITY OF FLORIDA 2003 ACKNOWLEDGMENTS I thank my committee members Lora Smith, Franklin Percival, and Dick Franz for all their support and advice. The Department of Interior's Student Career Experience Program and the U.S. Geological Survey's Amphibian Research and Monitoring Initiative provided funding for this project. I thank those involved with these programs who have helped me over the last three years: David Trauger, Ken Dodd, Jamie Barichivich, Jennifer Staiger, Kevin Smith, and Steve Johnson. Numerous people helped with field work: Audrey Owens, Maya Zacharow, Chris Gregory, Matt Chopp, Amanda Rice, Paul Loud, Travis Tuten, Steve Johnson, and Jennifer Staiger, Lora Smith, and the UF Wildlife Field Techniques Courses of2001-2002. Paul Moler and John Jensen provided advice and shared their wealth of herpetological knowledge. I thank the staff of Okefenokee National Wildlife Refuge and Steve Coates, manager of the Ordway Preserve, for their assistance on numerous occasions and for permission to conduct research on their property. Marinela Capanu of the IFAS Statistical Consulting Unit assisted with statistical analysis. Julien Martin, Bob Dorazio, Rob Bennets, and Cathy Langtimm provided advice on population analysis. I also thank the administrative staff of the Florida Caribbean Science Center and the Florida Cooperative Fish and Wildlife Research Unit. I am much indebted to all of these people, without whom this thesis would not have been possible. -

The Salamanders of Tennessee
Salamanders of Tennessee: modified from Lisa Powers tnwildlife.org Follow links to Nongame The Salamanders of Tennessee Photo by John White Salamanders are the group of tailed, vertebrate animals that along with frogs and caecilians make up the class Amphibia. Salamanders are ectothermic (cold-blooded), have smooth glandular skin, lack claws and must have a moist environment in which to live. 1 Amphibian Declines Worldwide, over 200 amphibian species have experienced recent population declines. Scientists have reports of 32 species First discovered in 1967, the golden extinctions, toad, Bufo periglenes, was last seen mainly species of in 1987. frogs. Much attention has been given to the Anurans (frogs) in recent years, however salamander populations have been poorly monitored. Photo by Henk Wallays Fire Salamander - Salamandra salamandra terrestris 2 Why The Concern For Salamanders in Tennessee? Their key role and high densities in many forests The stability in their counts and populations Their vulnerability to air and water pollution Their sensitivity as a measure of change The threatened and endangered status of several species Their inherent beauty and appeal as a creature to study and conserve. *Possible Factors Influencing Declines Around the World Climate Change Habitat Modification Habitat Fragmentation Introduced Species UV-B Radiation Chemical Contaminants Disease Trade in Amphibians as Pets *Often declines are caused by a combination of factors and do not have a single cause. Major Causes for Declines in Tennessee Habitat Modification -The destruction of natural habitats is undoubtedly the biggest threat facing amphibians in Tennessee. Housing, shopping center, industrial and highway construction are all increasing throughout the state and consequently decreasing the amount of available habitat for amphibians. -
I Online Supplementary Data – Sexual Size Dimorphism in Salamanders
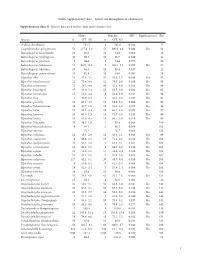
Online Supplementary data – Sexual size dimorphism in salamanders Supplementary data S1. Species data used in this study and references list. Males Females SSD Significant test Ref Species n SVL±SD n SVL±SD Andrias davidianus 2 532.5 8 383.0 -0.280 12 Cryptobranchus alleganiensis 53 277.4±5.2 52 300.9±3.4 0.084 Yes 61 Batrachuperus karlschmidti 10 80.0 10 84.8 0.060 26 Batrachuperus londongensis 20 98.6 10 96.7 -0.019 12 Batrachuperus pinchonii 5 69.6 5 74.6 0.070 26 Batrachuperus taibaiensis 11 92.9±12.1 9 102.1±7.1 0.099 Yes 27 Batrachuperus tibetanus 10 94.5 10 92.8 -0.017 12 Batrachuperus yenyuadensis 10 82.8 10 74.8 -0.096 26 Hynobius abei 24 57.8±2.1 34 55.0±1.2 -0.048 Yes 92 Hynobius amakusaensis 22 75.4±4.8 12 76.5±3.6 0.014 No 93 Hynobius arisanensis 72 54.3±4.8 40 55.2±4.8 0.016 No 94 Hynobius boulengeri 37 83.0±5.4 15 91.5±3.8 0.102 Yes 95 Hynobius formosanus 15 53.0±4.4 8 52.4±3.9 -0.011 No 94 Hynobius fuca 4 50.9±2.8 3 52.8±2.0 0.037 No 94 Hynobius glacialis 12 63.1±4.7 11 58.9±5.2 -0.066 No 94 Hynobius hidamontanus 39 47.7±1.0 15 51.3±1.2 0.075 Yes 96 Hynobius katoi 12 58.4±3.3 10 62.7±1.6 0.073 Yes 97 Hynobius kimurae 20 63.0±1.5 15 72.7±2.0 0.153 Yes 98 Hynobius leechii 70 61.6±4.5 18 66.5±5.9 0.079 Yes 99 Hynobius lichenatus 37 58.5±1.9 2 53.8 -0.080 100 Hynobius maoershanensis 4 86.1 2 80.1 -0.069 101 Hynobius naevius 72.1 76.7 0.063 102 Hynobius nebulosus 14 48.3±2.9 12 50.4±2.1 0.043 Yes 96 Hynobius osumiensis 9 68.4±3.1 15 70.2±3.0 0.026 No 103 Hynobius quelpaertensis 41 52.5±3.8 4 61.3±4.1 0.167 Yes 104 Hynobius -

Streamside Salamander Identification Guide
Key to the Identification of Streamside Salamanders 1 Ambystoma spp., mole salamanders (Family Ambystomatidae) Appearance: Medium to large stocky salamanders. Large round heads with bulging eyes. Larvae are also stocky and have elaborate gills. Size: 3-8” (Total length). Spotted salamander, Ambystoma maculatum Habitat: Burrowers that spend much of their life below ground in terrestrial habitats. Some species, (e.g. marbled salamander) may be found under logs or other debris in riparian areas. All species breed in fishless isolated ponds or wetlands. Range: Statewide. Other: Five species in Georgia. This group includes some of the largest and most dramatically patterned terrestrial species. Marbled salamander, Ambystoma opacum Amphiuma spp., amphiuma (Family Amphiumidae) Appearance: Gray to black, eel-like bodies with four greatly reduced, non-functional legs (A). Size: up to 46” (Total length) Habitat: Lakes, ponds, ditches and canals, one species is found in deep pockets of mud along the Apalachicola River floodplains. A Range: Southern half of the state. A Other: One species, the two-toed amphiuma (A. means), shown on the right, is known to occur in A. pholeter southern Georgia; a second species, ,Two-toed amphiuma, Amphiuma means may occur in extreme southwest Georgia, but has yet to be confirmed. The two-toed amphiuma (shown in photo) has two diminutive toes on each of the front limbs. 2 Cryptobranchus alleganiensis, hellbender (Family Cryptobranchidae) Appearance: Very large, wrinkled salamander with eyes positioned laterally (A). Brown-gray in color with darker splotches Size: 12-29” (Total length) A Habitat: Large, rocky, fast-flowing streams. Often found beneath large rocks in shallow rapids. -

(Amphibia, Urodela) from the Late Jurassic of Qinglong, Hebei Province, China
RESEARCH ARTICLE A New Basal Salamandroid (Amphibia, Urodela) from the Late Jurassic of Qinglong, Hebei Province, China Jia Jia, Ke-Qin Gao* School of Earth and Space Sciences, Peking University, 5 Yiheyuan Road, Beijing, 100871, China * [email protected] a11111 Abstract A new salamandroid salamander, Qinglongtriton gangouensis (gen. et sp. nov.), is named and described based on 46 fossil specimens of juveniles and adults collected from the Upper Jurassic (Oxfordian) Tiaojishan Formation cropping out in Hebei Province, China. OPEN ACCESS The new salamander displays several ontogenetically and taxonomically significant fea- Citation: Jia J, Gao K-Q (2016) A New Basal tures, most prominently the presence of a toothed palatine, toothed coronoid, and a unique Salamandroid (Amphibia, Urodela) from the Late pattern of the hyobranchium in adults. Comparative study of the new salamander with previ- Jurassic of Qinglong, Hebei Province, China. PLoS ONE 11(5): e0153834. doi:10.1371/journal. ously known fossil and extant salamandroids sheds new light on the early evolution of the pone.0153834 Salamandroidea, the most species-diverse clade in the Urodela. Cladistic analysis places Editor: William Oki Wong, Institute of Botany, CHINA the new salamander as the sister taxon to Beiyanerpeton, and the two taxa together form the basalmost clade within the Salamandroidea. Along with recently reported Beiyanerpe- Received: December 11, 2015 ton from the same geological formation in the neighboring Liaoning Province, the discovery Accepted: April 2, 2016 of Qinglongtriton indicates that morphological disparity had been underway for the sala- Published: May 4, 2016 mandroid clade by early Late Jurassic (Oxfordian) time. Copyright: © 2016 Jia, Gao. -

Comparative Biology of Sperm Storage in Female Salamanders
THE JOURNAL OF EXPERIMENTAL ZOOLOGY 282:460-476 (1998) Comparative Biology of Sperm Storage in Female Salamanders DAVID M. SEVERN* AND ROSSANA BRIZZI' 'Department of Biology, Saint Mary's College, Notre Dame, Indiana 46556 ^Dipartimento di Biologia Animale e Genetica, Universita di Firenze, 50125 Firenze, Italia ABSTRACT Females in seven of the ten families of salamanders possess cloacal glands called spermathecae that store sperm. The annual cycle of sperm storage has been studied by light and electron microscopy in eight species representing five families. In these taxa, we recognized 14 characters associated with the spermathecae and traced their evolution on a phylogeny of sala• manders based upon other characters. The plasticity and phyletic significance of the spermathecal characters varied greatly. Plethodontids have complex spermathecae while other families possess simple spermathecae; thus, this character has phyletic value as well as being highly conserved within the Salamandroidea. Other characters, such as carbohydrate histochemistry, are highly plastic and show no obvious phyletic trends. The significance of some of these variable characters, such as duration of sperm storage, is apparent only after including in the analysis other aspects of the reproductive cycle, such as length of the mating season. Additional comparative studies, em• ploying the protocol used in this paper, will help further clarify the relationships between phyletic and functional variability in sperm storage mechanisms in salamanders. J. Exp. Zool. 282:460- -

Sexual Dimorphism in the Three-Toed Amphiuma, Amphiuma Tridactylum: Sexual Selection Or Ecological Causes?
Copeia 2008, No. 1, 39–42 Sexual Dimorphism in the Three-toed Amphiuma, Amphiuma tridactylum: Sexual Selection or Ecological Causes? Clifford L. Fontenot, Jr.1 and Richard A. Seigel2 Sexual dimorphism is widespread among vertebrates, and may be attributable to sexual selection, differences in ecology between the sexes, or both. The large aquatic salamander, Amphiuma tridactylum, has been suggested to have male biased sexual dimorphism that is attributable to male–male combat, although detailed evidence is lacking. To test this, data were collected on A. tridactylum head and body size, as well as on bite-marks inflicted by conspecifics. Amphiuma tridactylum is sexually dimorphic in several characters. There was no sex difference in body length, but males had heavier bodies than females of the same body length. Larger males had wider and longer heads than larger females, but whether any of these sexually dimorphic characters are attributable to differences in diet is unknown because diet data (by sex) are lacking. There was no difference in the number of bite-marks between males and females, and juveniles also possessed bite-marks, suggesting that the biting is not necessarily related to courtship or other reproductive activities. In addition, fresh bite-marks were present on individuals during months well outside of the breeding season. Biting was observed in the field and lab to occur by both sexes on both sexes, during feeding-frenzy type foraging. Thus, biting is likely related to foraging rather than to courtship. The sexually dimorphic characters remain unexplained, pending sex-specific diet data, but there is no evidence that they are related to male–male combat or to courtship. -

WILDLIFE in a CHANGING WORLD an Analysis of the 2008 IUCN Red List of Threatened Species™
WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ Edited by Jean-Christophe Vié, Craig Hilton-Taylor and Simon N. Stuart coberta.indd 1 07/07/2009 9:02:47 WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ first_pages.indd I 13/07/2009 11:27:01 first_pages.indd II 13/07/2009 11:27:07 WILDLIFE IN A CHANGING WORLD An analysis of the 2008 IUCN Red List of Threatened Species™ Edited by Jean-Christophe Vié, Craig Hilton-Taylor and Simon N. Stuart first_pages.indd III 13/07/2009 11:27:07 The designation of geographical entities in this book, and the presentation of the material, do not imply the expressions of any opinion whatsoever on the part of IUCN concerning the legal status of any country, territory, or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. The views expressed in this publication do not necessarily refl ect those of IUCN. This publication has been made possible in part by funding from the French Ministry of Foreign and European Affairs. Published by: IUCN, Gland, Switzerland Red List logo: © 2008 Copyright: © 2009 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Citation: Vié, J.-C., Hilton-Taylor, C.