The Colour Violet: Chemistry Or Physics? (See Later)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Absorption of Light Energy Light, Energy, and Electron Structure SCIENTIFIC
Absorption of Light Energy Light, Energy, and Electron Structure SCIENTIFIC Introduction Why does the color of a copper chloride solution appear blue? As the white light hits the paint, which colors does the solution absorb and which colors does it transmit? In this activity students will observe the basic principles of absorption spectroscopy based on absorbance and transmittance of visible light. Concepts • Spectroscopy • Visible light spectrum • Absorbance and transmittance • Quantized electron energy levels Background The visible light spectrum (380−750 nm) is the light we are able to see. This spectrum is often referred to as “ROY G BIV” as a mnemonic device for the order of colors it produces. Violet has the shortest wavelength (about 400 nm) and red has the longest wavelength (about 650–700 nm). Many common chemical solutions can be used as filters to demonstrate the principles of absorption and transmittance of visible light in the electromagnetic spectrum. For example, copper(II) chloride (blue), ammonium dichromate (orange), iron(III) chloride (yellow), and potassium permanganate (red) are all different colors because they absorb different wave- lengths of visible light. In this demonstration, students will observe the principles of absorption spectroscopy using a variety of different colored solutions. Food coloring will be substituted for the orange and yellow chemical solutions mentioned above. Rare earth metal solutions, erbium and praseodymium chloride, will be used to illustrate line absorption spectra. Materials Copper(II) chloride solution, 1 M, 85 mL Diffraction grating, holographic, 14 cm × 14 cm Erbium chloride solution, 0.1 M, 50 mL Microchemistry solution bottle, 50 mL, 6 Potassium permanganate solution (KMnO4), 0.001 M, 275 mL Overhead projector and screen Praseodymium chloride solution, 0.1 M, 50 mL Red food dye Water, deionized Stir rod, glass Beaker, 250-mL Tape Black construction paper, 12 × 18, 2 sheets Yellow food dye Colored pencils Safety Precautions Copper(II) chloride solution is toxic by ingestion and inhalation. -

Light and the Electromagnetic Spectrum
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © JonesLight & Bartlett and Learning, LLCthe © Jones & Bartlett Learning, LLC NOTElectromagnetic FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION4 Spectrum © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALEJ AMESOR DISTRIBUTIONCLERK MAXWELL WAS BORN IN EDINBURGH, SCOTLANDNOT FOR IN 1831. SALE His ORgenius DISTRIBUTION was ap- The Milky Way seen parent early in his life, for at the age of 14 years, he published a paper in the at 10 wavelengths of Proceedings of the Royal Society of Edinburgh. One of his first major achievements the electromagnetic was the explanation for the rings of Saturn, in which he showed that they con- spectrum. Courtesy of Astrophysics Data Facility sist of small particles in orbit around the planet. In the 1860s, Maxwell began at the NASA Goddard a study of electricity© Jones and & magnetismBartlett Learning, and discovered LLC that it should be possible© Jones Space & Bartlett Flight Center. Learning, LLC to produce aNOT wave FORthat combines SALE OR electrical DISTRIBUTION and magnetic effects, a so-calledNOT FOR SALE OR DISTRIBUTION electromagnetic wave. -

10 Comparing Colors LABORATORY 1 C L a S S S E S S I O N
10 Comparing Colors LABORATORY 1 CLASS SESSION ACTIVITY OVERVIEW NGSS RATIONALE In this activity, students first learn that visible light can be separated into different colors. Students then conduct an investigation to collect evidence that indicates that different colors of light carry different amounts of energy. From the results of their investigation, students conclude that higher frequencies of light carry more energy. In their final analysis, students apply the practice of analyzing and inter- preting data as they examine light transmission graphs for three different sunglass lenses. It is through this analysis that students engage with the crosscutting con- cept of structure and function as they determine which sunglass lens provides the best protection for the eyes. NGSS CORRELATION Disciplinary Core Ideas PS-4.B Wave Properties: When light shines on an object, it is reflected, absorbed, or transmitted through the object, depending on the object’s material and the frequency (color) of the light. PS-4.B Wave Properties: A wave model of light is useful for explaining brightness, color, and the frequency-dependent bending of light at a surface between media. Science and Engineering Practices Planning and Carrying Out Investigations: Conduct an investigation to produce data to serve as the basis for evidence that meets the goals of an investigation. Crosscutting Concepts Structure and Function: Structures can be designed to serve particular functions by taking into account properties of different materials, and how materials can be shaped and used. WAVES 3 ACTIVITY 10 COMPARING COLORS Common Core State Standards—ELA/Literacy RST.6-8.3 Follow precisely a multi-step procedure when carrying out experiments, taking measurements, or performing technical tasks. -

Why Is the Sky Purple?
A laboratory experiment from the Why is Little Shop of Physics at Colorado State University the sky CMMAP purple? Reach for the sky. Overview Necessary materials: Of course, you expect the question to be “why is the sky blue?” That’s the classic version. • 1 “sunset egg” • A white light flashlight And here’s the classic answer: scattering. We’ll talk about what this word means and how it leads to sky color, but we will also see that the The most crucial piece for this experiment is the light from the sky actually contains a bit more “sunset egg.” The small-scale structure of these violet than it does blue! So why do we see glori- glass “eggs” works well to demonstrate the differ- ous blue skies rather than a purple firmament ential scattering that leads to the color of the sky when we gaze up into Earth’s atmosphere? and the color of the sunset. Theory You can find them at rock and nature shops, or you can purchase them in bulk from Pelham Grayson The first person to correctly work out the de- (www.pelhamgrayson.com) under “Magic Feng tails of the process that gives rise to the color of Shui Eggs”. the sky was the English physicist, Lord John Rayleigh, working in the late 1800’s. Rayleigh correctly surmised that the blue color of the sky was a result of scattering. As light enters our atmosphere on its journey from the sun, it interacts with air molecules and is redirected. This redirection is more pronounced for shorter wavelengths toward the blue, or violet, end of the spectrum. -

The Electromagnetic Spectrum CESAR’S Booklet
The electromagnetic spectrum CESAR’s Booklet The electromagnetic spectrum The colours of light You have surely seen a rainbow, and you are probably familiar with the explanation to this phenomenon: In very basic terms, sunlight is refracted as it gets through water droplets suspended in the Earth’s atmosphere. Because white light is a mixture of six (or seven) different colours, and each colour is refracted a different angle, the result is that the colours get arranged in a given order, from violet to red through blue, green, yellow and orange. We can get the same effect in a laboratory by letting light go through a prism, as shown in Figure 1. This arrangement of colours is what we call a spectrum. Figure 1: White light passing through a prism creates a rainbow. (Credit: physics.stackexchange.com) Yet the spectrum of light is not only made of the colours we see with our eyes. There are other colours that are invisible, although they can be detected with the appropriate devices. Beyond the violet, we have ultraviolet, X-rays and gamma rays. On the other extreme, beyond the red, we have infrared and radio. Although we cannot see them, we are familiar with these other types of light: for example, we use radio waves to transmit music from one station to our car receiver, ultraviolet light from the Sun makes our skin get tanned, X-rays are used by radiography machines to check if we have a broken bone, or we change channel in our TV device by sending an infrared signal to it from the remote control. -

The Relationship Between Wavelength and Frequency in the Electromagnetic Spectrum
Name_______________________________________ The Relationship Between Wavelength and Frequency in the Electromagnetic Spectrum Purpose: To discover and verify the relationship between Wavelength and Frequency of the Electromagnetic Spectrum. Background Information: Visible light is Electromagnetic radiation at wavelengths which the human eye can see. We perceive this radiation as colors ranging from red (longer wavelengths; ~ 700 nanometers) to violet (shorter wavelengths; ~400 nanometers.) The visible light from the Sun is actually composed of the colors red, orange, yellow, green, blue, and violet, which can become distinguishable when sunlight passes through a prism. A good way to remember the order of the colors is to note that the first letters of the colors spell out the name ROY G. BV. We can think of light traveling in waves with properties of wavelength and frequency. Wavelength is the distance between identical locations on adjacent waves (see figure below). Frequency is the number of complete waves, or wavelengths, that pass a given point each second. All light travels at the same speed, but each color has a different wavelength and frequency. It is their different wavelengths that cause the different colors of light to separate and become visible when passing through a prism. Look at the illustration of the visible spectrum above. Can you guess which color has the longest wavelength? It's red! The wavelengths of the other colors decrease in order, with violet light having the shortest. Adapted from: http://imagine.gsfc.nasa.gov/docs/teachers/lessons/roygbiv/roygbiv.html Before you start: Summarize the graphic on the electromagnetic spectrum shown below. Think about the relationships in and among the various wavelengths and the position of each type of radiation in the electromagnetic spectrum. -

AOA's Light and Eye Damage
Light and Eye Damage Gregory W. Good, O.D., Ph.D. Light and Eye Damage · Gregory W. Good, O.D., Ph.D. Questions: 1. WHAT SPECIFIC PORTIONS OF THE ENVIRONMENTAL ELECTROMAGNETIC SPECTRUM CAUSE EYE DAMAGE? 2. IS THERE ANY EVIDENCE TO SUGGEST THAT BLUE LIGHT/UV RADIATION CAUSES SIGNIFICANT DAMAGE TO THE EYE? 3. AT WHAT AGE SHOULD AN OPTOMETRIST RECOMMEND PROTECTIVE EYEWEAR COATINGS AND CONTACT LENSES WITH UV RADIATION AND VISIBLE LIGHT PROTECTION? 4. WHAT ARE THE OPHTHALMIC MATERIALS THAT PROTECT EYES FROM CERTAIN LIGHT SOURCES? 5. WHAT DO THE LABELS “SPF” OR “UV” MEAN ON OTC SUNGLASSES BEING SOLD? 6. WHAT GUIDELINES, IF ANY, SHOULD BE INCLUDED IN INFANTSEE® MATERIALS REGARDING PROTECTIVE EYEWEAR FOR CHILDREN (SOMETHING TO PROVIDE TO PARENTS WHEN THEY LEAVE THE OFFICE)? 7. CONTACT LENSES AND UV PROTECTION 8. WHAT IS THE BEST LIGHT SOURCE FOR READING, WATCHING TV, COMPUTER AND TABLET USE, ETC.? 9. WHAT ARE THE EFFECTS OF BLUE LIGHT ON SLEEP PATTERNS AND MELATONIN? 10. WHAT ARE THE EYE HEALTH RELATED CONCERNS FOR PATIENTS EXPOSED TO LIGHT FROM OPHTHALMIC EQUIPMENT, SUCH AS: BIO, SLIT LAMPS, DO, ETC.? 11. WHAT LIGHTING IS RECOMMENDED FOR SAD OR DEPRESSION THAT DOES NOT HAVE ADVERSE EFFECTS TO THE EYE? 12. WHAT ARE THE EYE HEALTH CONCERNS FOR DENTAL STAFF AND PATIENTS EXPOSED TO LIGHT FROM DENTAL EQUIPMENT, E.G. LASER WHITENING? December 2014 Page 2 Light and Eye Damage · Gregory W. Good, O.D., Ph.D. 1. WHAT SPECIFIC PORTIONS OF THE ENVIRONMENTAL ELECTROMAGNETIC SPECTRUM CAUSE EYE DAMAGE? With sufficient magnitude almost all portions of the electromagnetic spectrum can cause damage to the eye. -

The Science of Color
The Science of Color Words to Know ● wavelength ● cellulose ● chromophore ● electromagnetic ● color ● covalent bond ● spectrum ● hue ● rainbow ● additive color ● saturation ● retina ● subtractive ● value ● alkali ● hydrogen bond ● hydroxyl group ● catalyst What is Color? What is color? What does it have to do with light? Color is an effect produced by the eye and its nerves by light waves of different wavelengths or frequencies. Light transmitted from an object to the eye stimulates the different color cones of the retina, making possible perception of various colors in the object. Since the colors that compose sunlight or white light have different wavelengths. Meaning, the speed at which they travel through a medium such as glass differs; red light, which has the longest wavelength, travels faster through glass than blue light, which has a shorter wavelength. When white light passes through a glass prism, it separates into bands of colors called a spectrum. The colors of the visible spectrum are usually called red, orange, yellow, green, blue, indigo, and violet. The phrase ROY-G-BIV is an easy way to remember this. This same effect happens when (sun)light goes through water droplets or rain causing us to see a rainbow. Our eyes are sensitive to light which lies in a very small region of the electromagnetic spectrum labeled "visible light." This “visible light" corresponds to a wavelength range of 400 - 700 nanometers (nm) and a color range of violet through red. The human eye is not capable of “seeing" radiation with wavelengths outside the visible spectrum (see figure B). How do Reactive Dyes Work? Dye is a powder, which dissolves in water. -

And Non-Ionizing Radiation Study Guide
Ionizing and Non-Ionizing Radiation Study Guide Radiation - Energy emitted from a body or source that is transmitted through an intervening medium or space and absorbed by another body. Transmission is in the form of waves but wave/particle duality under quantum physics. Radiation is classified as being either non-ionizing or ionizing . Non-ionizing radiation is longer wavelength/lower frequency lower energy. While ionizing radiation is short wavelength/high frequency higher energy. Ionizing Radiation has sufficient energy to produce ions in matter at the molecular level. If that matter is a human significant damage can result including damage to DNA and denaturation of proteins. This is not to say that non-ionizing radiation can’t cause injury to humans but the injury is generally limited to thermal damage i.e. burns. There is a great deal of information on the above chart. One of the most interesting things is that the visible spectrum is essentially the divide between ionizing and non-ionizing radiation. This makes sense clinically when we think of UV radiation causing skin cancer. 9 Types of Non-Ionizing Radiation and Their Clinical Effects - Referring again to the chart above we can see that Non-Ionizing radiation comes in the forms of: 1. ELF (extremely low frequency) 2. Radio Frequencies 3. Microwave Frequencies 4. Lasers 5. Infrared 6. Visible Spectrum 7. Ultraviolet This list is in order of lowest to highest frequency. 1. ELF Power plant or line workers Inconclusive evidence of leukemia link 2. 3. Radiofrequency and Microwave Frequency Exposures- Occupational Exposures- Radar and communications equipment, industrial and commercial ovens Other Exposures Cell Phones Clinical Effects- There is a great deal of controversy regarding potential cancer risks, particularly with cell phone use. -

Energy in Photons Light, Energy, and Electron Structure SCIENTIFIC
Energy in Photons Light, Energy, and Electron Structure SCIENTIFIC Introduction Students often confuse the concepts of intensity of light and energy of light. This demonstration provides a clear way to demonstrate that the intensity, or brightness, of light is NOT the same as the amount of energy a particular color of light possesses. Concepts • Phosphorescence • Absorbance • Transmittance • Emission Materials Energy in Photons Demonstrator Card—contains phosphorescent strip and light filters* Light source—classroom lights or overhead projector *Available in kit AP4576. Safety Precautions This activity is considered nonhazardous. Please follow all normal classroom safety guidelines. Procedure 1. Open the Demonstrator Card and show the class the phosphorescent strip. Explain to them that the common term for phosphorescence is “glow-in-the-dark” and that this strip will glow in the dark. 2. Directly expose the entire phosphorescent strip to the classroom lights for about 15 seconds. Now turn off all the classroom lights and completely darken the room. The entire strip will glow brightly for several minutes and then begin to fade. Once students are satisfied with the glow, turn the classroom lights back on. 3. Show the six colored filters on the Demonstrator Card to the class. Hold the Demonstrator Card up to the light so that the color of light transmitted through each filter is clearly visible. Observe that the color of light transmitted through each filter is the same color as the filter. 4. Have the class predict what will happen if the Demonstrator Card is closed so that only “filtered” light is allowed to shine upon the phosphorescent strip. -
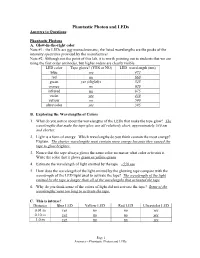
Phantastic Photon and Leds Answers to Questions
Phantastic Photon and LEDs Answers to Questions: Phantastic Photons A. Glow-in-the-right color Note #1: the LEDs are not monochromatic; the listed wavelengths are the peaks of the intensity spectrums provided by the manufacturer. Note #2: Although not the point of this lab, it is worth pointing out to students that we are using the first order antinodes, but higher orders are clearly visible. LED color Tape glows? (YES or NO) LED wavelength (nm) blue yes 472 red no 660 green yes (slightly) 525 orange no 620 infrared no 875 violet yes 430 yellow no 590 ultraviolet yes 395 B. Exploring the Wavelengths of Colors 1. What do you notice about the wavelengths of the LEDs that make the tape glow? The wavelengths that make the tape glow are all relatively short, approximately 530 nm and shorter. 2. Light is a form of energy. Which wavelengths do you think contain the most energy? Explain. The shorter wavelengths must contain more energy because they caused the tape to glow brighter. 3. Notice that the tape always glows the same color no matter what color activates it. Write the color that it glows green or yellow-green 4. Estimate the wavelength of light emitted by the tape _~550 nm 5. How does the wavelength of the light emitted by the glowing tape compare with the wavelength of the LED light used to activate the tape? The wavelength of the light emitted by the tape is longer than all of the wavelengths that activated the tape 6. Why do you think some of the colors of light did not activate the tape? Some of the wavelengths were too long to activate the tape. -

Indigo Blue EXPERIMENT: VISIBLE LIGHT SPECTROSCOPY in This
EXPERIMENT: VISIBLE LIGHT SPECTROSCOPY In this experiment we will be investigating the color produced when light is absorbed by a transparent solution. The color a solution will appear to us can be predicted by using the color wheel. If the chemicals in the solution absorb only red light, the solution will appear blue-green. Blue-green is the color directly opposite red on the color wheel. Colors opposite one another on the color wheel are called complementary colors. Red and blue-green are complementary colors. If a solution appears blue then we would find that the chemicals are absorbing orange light since blue and orange are complementary. It should be noted that there is no such thing as purple light (purple is red + violet ) so if a solution is green it can’t absorb “purple” light. Instead it absorbs reds and violets. Indigo Blue We would also expect that as the concentration of the absorbing chemicals increase the amount of light absorbed would probably increase also. This relationship is called “Beer’s Law.” The instrument we will use to determine the type of light absorbed by the solution and the amount of light absorbed is called a spectrometer. Verification of the Color Wheel Connect the spectrometer to the USB port of your computer and open Logger Pro. You should see this screen: Select Experiment from the toolbar and then Calibrate, Spectrometer from the menu. Fill a sample holder (called a cuvette) about 3/4 full of distilled water and position it into the spectrometer so that the rubbed sides of the cuvette do not interfere with the light beam.