The Science of Color
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Introduction to the Chemistry of Dyes
GENERAL INTRODUCTION TO THE CHEMISTRY OF DYES 1. Principles of Colour Chemistry 1.1 Basis for colour Unlike most organic compounds, dyes possess colour because they 1) absorb light in the visible spectrum (400–700 nm), 2) have at least one chromophore (colour-bearing group), 3) have a conjugated system, i.e. a structure with alternating double and single bonds, and 4) exhibit resonance of electrons, which is a stabilizing force in organic compounds (Abrahart, 1977). When any one of these features is lacking from the molecular structure the colour is lost. In addition to chromophores, most dyes also contain groups known as auxochromes (colour helpers), examples of which are carboxylic acid, sulfonic acid, amino, and hydroxyl groups. While these are not responsible for colour, their presence can shift the colour of a colourant and they are most often used to influence dye solubility. Figure 1 shows the relationships between wavelength of visible and colour absorbed/observed. Other factors contributing to colour are illustrated in Figures 2–4. Fig. 1. Wavelength of light absorption versus colour in organic dyes Wavelength Absorbed (nm) Colour Absorbed Colour Observed 400–435 Violet Yellow-Green 435–480 Blue Yellow 480–490 Green-Blue Orange 490–500 Blue-Green Red 500–560 Green Purple 560–580 Yellow-Green Violet 580–595 Yellow Blue 595–605 Orange Green-Blue 605–700 Red Blue-Green –55– 56 IARC MONOGRAPHS VOLUME 99 Fig. 2. Examples of chromophoric groups present in organic dyes O O N N N N O N N O H N Anthraquinone H N Nitro Azo N N Ar C N N C Ar Methine Phthalocyanine Triarylmethane Fig. -

Identification and Distribution of Dietary Precursors of The
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Vision Research 39 (1999) 219–229 Identification and distribution of dietary precursors of the Drosophila visual pigment chromophore: analysis of carotenoids in wild type and ninaD mutants by HPLC David R. Giovannucci *, Robert S. Stephenson Department of Biological Sciences, Wayne State Uni6ersity, Detroit, MI 48202, USA Received 3 July 1997; received in revised form 10 November 1997 Abstract A dietary source of retinoid or carotenoid has been shown to be necessary for the biosynthesis of functional visual pigment in flies. In the present study, the larvae or adults of Drosophila melanogaster were administered specific carotenoid-containing diets and high performance liquid chromatography was used to identify and quantify the carotenoids in extracts of wild type and ninaD visual mutant flies. When b-carotene was fed to larvae, wild type flies were shown to hydroxylate this molecule and to accumulate zeaxanthin and a small amount of b-cryptoxanthin. Zeaxanthin content was found to increase throughout development and was a major carotenoid peak detected in the adult fly. Carotenoids were twice as effective at mediating zeaxanthin accumulation when provided to larvae versus adults. In the ninaD mutant, zeaxanthin content was shown to be specifically and significantly altered compared to wild type, and was ineffective at mediating visual pigment synthesis when provided to both larval and adult mutant flies. It is proposed that zeaxanthin is the larval storage form for subsequent visual pigment chromophore biosynthesis during pupation, that zeaxanthin or b-crytoxanthin is the immediate precursor for light-independent chromophore synthesis in the adult, and that the ninaD mutant is defective in this pathway. -

Absorption of Light Energy Light, Energy, and Electron Structure SCIENTIFIC
Absorption of Light Energy Light, Energy, and Electron Structure SCIENTIFIC Introduction Why does the color of a copper chloride solution appear blue? As the white light hits the paint, which colors does the solution absorb and which colors does it transmit? In this activity students will observe the basic principles of absorption spectroscopy based on absorbance and transmittance of visible light. Concepts • Spectroscopy • Visible light spectrum • Absorbance and transmittance • Quantized electron energy levels Background The visible light spectrum (380−750 nm) is the light we are able to see. This spectrum is often referred to as “ROY G BIV” as a mnemonic device for the order of colors it produces. Violet has the shortest wavelength (about 400 nm) and red has the longest wavelength (about 650–700 nm). Many common chemical solutions can be used as filters to demonstrate the principles of absorption and transmittance of visible light in the electromagnetic spectrum. For example, copper(II) chloride (blue), ammonium dichromate (orange), iron(III) chloride (yellow), and potassium permanganate (red) are all different colors because they absorb different wave- lengths of visible light. In this demonstration, students will observe the principles of absorption spectroscopy using a variety of different colored solutions. Food coloring will be substituted for the orange and yellow chemical solutions mentioned above. Rare earth metal solutions, erbium and praseodymium chloride, will be used to illustrate line absorption spectra. Materials Copper(II) chloride solution, 1 M, 85 mL Diffraction grating, holographic, 14 cm × 14 cm Erbium chloride solution, 0.1 M, 50 mL Microchemistry solution bottle, 50 mL, 6 Potassium permanganate solution (KMnO4), 0.001 M, 275 mL Overhead projector and screen Praseodymium chloride solution, 0.1 M, 50 mL Red food dye Water, deionized Stir rod, glass Beaker, 250-mL Tape Black construction paper, 12 × 18, 2 sheets Yellow food dye Colored pencils Safety Precautions Copper(II) chloride solution is toxic by ingestion and inhalation. -

Primary and Secondary Colors Secondary and Primary Science Language Arts Camouflaging Colors
Science Primary and Secondary Colors Name Red, yellow, and blue are primary colors. Orange, green, and purple are secondary colors. A secondary color is created by mixing together two primary colors. By adding the color white, you can make all of these colors a shade lighter. Color each paint splotch with water-based markers. Make sure you color the whole splotch. What new colors did you create? 1. Yellow and Blue 2. Red and Yellow Color: Color: 3. Red and Blue 4. Red, Orange, Yellow, Green, Blue, Purple Color: Color: Now color these splotches with the following crayons: 5. Blue and White 6. Red and White Answers: 1. green 2. orange 3. purple 4. black 5. light blue 6. pink 6. blue light 5. black 4. purple 3. orange 2. green 1. Answers: © Learning Resources, Inc. Language Arts Camouflaging Colors Name Being camouflaged is a good way to stay safe. Many animals can change their colors, or camouflage themselves, to blend in with their surroundings. Chameleons and frogs are good examples of animals that are hard to find in their habitats. Think about where Carl Chameleon might live. Add in his surroundings, and then use your crayons to camouflage him in his environment. What color would he be? Think about how you would camouflage yourself in your bedroom. What kinds of clothes or face paint would you have to wear? © Learning Resources, Inc. Language Arts Color Matching Name Match the object to its color. Then use crayons to color each picture. 1. white 2. pink yellow 3. 4. red 5. -

Ba3(P1−Xmnxo4)2 : Blue/Green Inorganic Materials Based on Tetrahedral Mn(V)
Bull. Mater. Sci., Vol. 34, No. 6, October 2011, pp. 1257–1262. c Indian Academy of Sciences. Ba3(P1−xMnxO4)2 : Blue/green inorganic materials based on tetrahedral Mn(V) SOURAV LAHA, ROHIT SHARMA, S V BHAT†, MLPREDDY‡, J GOPALAKRISHNAN∗ and S NATARAJAN Solid State and Structural Chemistry Unit, †Department of Physics, Indian Institute of Science, Bangalore 560 012, India ‡Chemical Science and Technology Division, National Institute for Interdisciplinary Science and Technology (NIIST), Thiruvananthapuram 695 019, India MS received 11 May 2011 ) 3− 2 Abstract. We describe a blue/green inorganic material, Ba3(P1−xMnxO4 2 (I) based on tetrahedral MnO4 :3d chromophore. The solid solutions (I) which are sky-blue and turquoise-blue for x ≤ 0·25 and dark green for x ≥ 0·50, 3− are readily synthesized in air from commonly available starting materials, stabilizing the MnO4 chromophore in an isostructural phosphate host. We suggest that the covalency/ionicity of P–O/Mn–O bonds in the solid solutions tunes the crystal field strength around Mn(V) such that a blue colour results for materials with small values of x. The material could serve as a nontoxic blue/green inorganic pigment. Keywords. Blue/green inorganic material; barium phosphate/manganate(V); tetrahedral manganate(V); blue/green chromophore; ligand field tuning of colour. 1. Introduction field transitions within an unusual five coordinated trigo- nal bipyramidal Mn(III). A turquoise-blue solid based on Inorganic solids displaying bright colours are important Li1·33Ti1·66O4 spinel oxide wherein the colour arises from as pigment materials which find a wide range of appli- an intervalence charge-transfer between Ti3+ and Ti4+ has cations in paints, inks, plastics, rubbers, ceramics, ena- also been described recently (Fernández-Osorio et al 2011). -
Color Vision Light
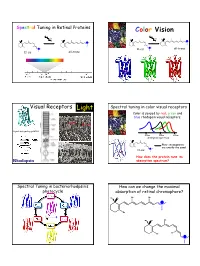
Spectral Tuning in Retinal Proteins Color Vision hν hν N N H H H H N all-trans N 11-cis 11-cis all-trans Visual Receptors Light Spectral tuning in color visual receptors Color is sensed by red, green and blue rhodopsin visual receptors. Rod Cone G-protein signaling pathway 400nm 500nm 600nm absorption spectrum Their chromophores H are exactly the same! 11-cis N How does the protein tune its Rhodopsin absorption spectrum? Spectral Tuning in bacteriorhodpsin’s How can we change the maximal photocycle absorption of retinal chromophore? Me Me Me Me N H Me Me Me Me N H Me Me Me Me Me Me Me H N 1 Excitation energy determines the Electronic Absorption maximal absorption Me Me Me Me π π* N - H S1 Me Absorption of light in the UV-VIS region of the S0 spectrum is due to Response excitation of electrons to higher energy levels. Me Me Me 13 7911 15 N H Me Me π-π* excitation in polyenes π-π* excitation in polyenes π∗ π∗ π∗ π∗ ∆E π∗ E photon E π π π π π Ground state (S0) Excited state (S1) ∆E (excitation energy, band gap) = hν = hc/λ blue-shift red-shift π-π* excitation in polyenes Tuning the length of the conjugated backbone β-carotene O O Vitamin A2 (retinal II) Vitamin A1 (retinal I) Longer wavelength Short wavelength 2 Retinal I Retinal II OPSIN SHIFT: how protein tunes the absorption maximum of its chromophore. Maximal absorption of protonated retinal Schiff base in: Water/methanol solution: 440 nm bR: 568 nm rod Rh: 500 nm red receptor: 560 nm green receptor: 530 nm blue receptor: 426 nm Salmon: different retinals in different stages of life Electrostatics and opsin shift Electrostatics and opsin shift S S positive charge 2 positive charge 2 S2 S2 Me Me Me Me Me Me S1 S1 S1 S1 + + + + N N H H Me O S0 Me O S0 Me O Me O C C Asp (Glu) Asp (Glu) S0 S0 no protein in protein no protein in protein counterion counterion • The counterion stabilizes the positive charge of the chromophore. -

My Art Adventure Rv 2
Understanding The Color Wheel What is a color wheel? A color wheel is a painting tool artists use to help understand the relationships between individual colors and how to best use them. By name, each color is as follows: red, red-purple, purple, blue-purple, blue, blue-green, green, yellow-green, yellow, yellow-orange, orange, and orange red. Sometimes, colors have more common names like “turquoise” for blue-green, and “magenta” for red-purple. Primary Colors Primary colors are the building blocks that make all the other colors on the wheel. Here on our color diagram we can see the 3 primary colors. We know them as red, yellow, and blue. Fun fact:Did you know that you can create ANY color you need from mixing red, yellow, or blue paint? The primary colors on the color wheel are the most powerful colors. Yellow is the brightest color on the wheel while red and blue have been known as “power colors”. That’s why fast-food restaurants like McDonald’s use red and yellow in its logo - so you can see it from far away! Secondary Colors A secondary color is a combination of 2 primary colors. There are 3 secondary colors on our wheel - green, orange and purple. Here is a summary of how to create the secondary colors: Tertiary Colors Tertiary colors are the last addition to our wheel. Tertiary colors are a mixture of a primary color and a secondary color. Each tertiary color is named from a combination of the primary and secondary colors, like yellow- green. -

Indigo Chromophores and Pigments Structure and Dynamics
Dyes and Pigments 172 (2020) 107761 Contents lists available at ScienceDirect Dyes and Pigments journal homepage: www.elsevier.com/locate/dyepig Indigo chromophores and pigments: Structure and dynamics T ∗ V.V. Volkova, R. Chellib, R. Righinic, C.C. Perrya, a Interdisciplinary Biomedical Research Centre, School of Science and Technology, Nottingham Trent University, Clifton Lane, Nottingham, NG11 8NS, United Kingdom b Dipartimento di Chimica, Università degli Studi di Firenze, Via della Lastruccia 3, Sesto Fiorentino, 50019, Italy c European Laboratory for Non-Linear Spectroscopy (LENS), Università degli Studi di Firenze, Via Nello Carrara 1, Sesto Fiorentino, 50019, Italy ARTICLE INFO ABSTRACT Keywords: In this study, we explore the molecular mechanisms of the stability of indigo chromophores and pigments. Indigo Assisted with density functional theory, we compare visible, infrared and Raman spectral properties of model Raman molecules, chromophores and pigments derived from living organisms. Using indigo carmine as a representative Infrared model system, we characterize the structure and dynamics of the chromophore in the first electronic excited Density functional theory state using femtosecond visible pump-infrared probe spectroscopy. Results of experiments and theoretical stu- Excited state dies indicate that, while the trans geometry is strongly dominant in the electronic ground state, upon photo- excitation, in the Franck-Condon region, some molecules may experience isomerization and proton transfer dynamics. If this happens, however, the normal modes of the trans geometry of the electronic excited state are reconfirmed within several hundred femtoseconds. Supported by quantum theory, first, we ascribe stabilization of the trans geometry in the Franck-Condon region to the reactive character of the potential energy surface for the indigo chromophore when under the cis geometry in the electronic excited state. -

Blinded by the Light
Islands in the Stream 2002: Exploring Underwater Oases Blinded by the Light FOCUS 1 piece of blue color filter or plastic wrap Reflection, absorption, and scattering of light in the 1 piece of green color filter or plastic wrap ocean 1 piece of magenta* filter 1 piece of cyan* filter GRADE LEVEL 1 piece of yellow* filter 9-12 (Physical Science) 1 red marker 1 blue marker FOCUS QUESTION 1 green marker How is it possible for a fish to look one color in deep water and a different color above the water 1 yellow marker in bright sunlight? 8” x 11” white copy paper, 3 pieces per group of students LEARNING OBJECTIVES 1 red apple Students will recognize that the colors they see are 1 green apple a result of the reflection of light and that other col- 1 banana ors of light are absorbed. 1 blueberry 1 lime Students will predict what color an object will Any other colored fruit/object appear when light of different colors is shined upon * If you do not have access to these filters in it. your physics laboratory, they can be purchased from Arbor Scientific, POB 2750, Ann Arbor, MI Students will predict what color(s) will be produced 48106-2750, 1.800.367.6695 (Product ID 33- when different colors of light are mixed. 0190, Category Light and Color, Color Filters Kit, Students will identify the three primary colors and $12.00) three secondary colors of light. A/V MATERIALS ADDITIONAL INFORMATION FOR TEACHERS OF DEAF STUDENTS None Words listed as key words have no formal signs in American Sign Language and many are difficult to TEACHING TIME lipread. -

Color Schemes Are Combinations of Colors
Color is the reflection of light off of an object into our eyes. Our eyes then read the speed of the light and tell us which color that object is. There are two major categories under the heading of color, they are: 1. Neutrals 2. Colors Neutrals are (combinations of) black and white and all grays Colors consist of: Primary colors Secondary colors Intermediate colors also known as Tertiary colors Primary Colors: are the basic colors that you cannot make by mixing. They are natural colors found in nature. They are red, yellow, and blue. Secondary Colors: are made by mixing any two secondary colors. The secondary colors are orange, violet and green. Intermediate Colors: are made by mixing a primary and a secondary color. The secondary colors are, red-violet, blue-violet, blue-green, yellow-green, yellow-orange and red-orange. Color schemes are combinations of colors. There are many different types of color combinations, however, only four of the most basic are included here. They are: • Complementary colors • Analogous colors • Warm & Cool colors • Monochromatic colors Complementary Colors: are any two colors that are opposite each other on the color wheel. Analogous Colors: are any two colors that are adjacent to (or next to) each other on the color wheel. Warm & Cool Colors: warm colors are those colors that contain combinations of red and yellow. There are six. To help you remember what a warm color is, think of the sun or fire. Cool colors are those colors that contain green and blue. There are six of these too. -

Molecular Evolution of Color Vision in Vertebrates
Gene 300 (2002) 69–78 www.elsevier.com/locate/gene Molecular evolution of color vision in vertebrates Shozo Yokoyama* Department of Biology, Biological Research Laboratories, Syracuse University, 130 College Place, Syracuse, NY 13244, USA Received 10 December 2001; received in revised form 4 March 2002; accepted 17 July 2002 Abstract Visual systems of vertebrates exhibit a striking level of diversity, reflecting their adaptive responses to various color environments. The photosensitive molecules, visual pigments, can be synthesized in vitro and their absorption spectra can be determined. Comparing the amino acid sequences and absorption spectra of various visual pigments, we can identify amino acid changes that have modified the absorption spectra of visual pigments. These hypotheses can then be tested using the in vitro assay. This approach has been a powerful tool in elucidating not only the molecular bases of color vision, but the processes of adaptive evolution at the molecular level. q 2002 Elsevier Science B.V. All rights reserved. Keywords: Visual pigment; Wavelength absorption; Color vision; Vertebrate 1. Introduction visual pigments, it was necessary to clone opsin genes. This was initiated in 1986 when the opsin genes were cloned Vision has profound effects on the evolution of from cow and human (Nathans and Hogness, 1983, 1984; organisms by affecting survivorship through such behaviors Nathans et al., 1986). The availability of these opsin clones as mating, foraging, and predator avoidance. The data led to the isolation of other orthologous and paralogous collected by vision scientists over the last century genes from various species. Second, in the late 1980s, it demonstrate beyond any doubt that ecology has been a became possible to express opsins in cultured cells, major factor in directing the evolution of visual systems reconstitute with 11-cis-retinal, and measure the absorption (Walls, 1942; Lythgoe, 1979; Jacobs, 1981; Bowmaker, spectra of the resulting visual pigments in vitro (Oprian 1991; Yokoyama and Yokoyama, 1996). -

Light and the Electromagnetic Spectrum
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © JonesLight & Bartlett and Learning, LLCthe © Jones & Bartlett Learning, LLC NOTElectromagnetic FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION4 Spectrum © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALEJ AMESOR DISTRIBUTIONCLERK MAXWELL WAS BORN IN EDINBURGH, SCOTLANDNOT FOR IN 1831. SALE His ORgenius DISTRIBUTION was ap- The Milky Way seen parent early in his life, for at the age of 14 years, he published a paper in the at 10 wavelengths of Proceedings of the Royal Society of Edinburgh. One of his first major achievements the electromagnetic was the explanation for the rings of Saturn, in which he showed that they con- spectrum. Courtesy of Astrophysics Data Facility sist of small particles in orbit around the planet. In the 1860s, Maxwell began at the NASA Goddard a study of electricity© Jones and & magnetismBartlett Learning, and discovered LLC that it should be possible© Jones Space & Bartlett Flight Center. Learning, LLC to produce aNOT wave FORthat combines SALE OR electrical DISTRIBUTION and magnetic effects, a so-calledNOT FOR SALE OR DISTRIBUTION electromagnetic wave.