Nilaparvata Lugens, Stal) Population Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Defense and Counter-Defense in Rice–Virus Interactions Jiaqi Qin, Ci Wang, Leqi Wang, Shanshan Zhao* and Jianguo Wu*
Qin et al. Phytopathology Research (2019) 1:34 https://doi.org/10.1186/s42483-019-0041-7 Phytopathology Research REVIEW Open Access Defense and counter-defense in rice–virus interactions Jiaqi Qin, Ci Wang, Leqi Wang, Shanshan Zhao* and Jianguo Wu* Abstract Rice viruses, known as “rice killer”, are vector-borne pathogens that cause severe disease and significant yield loss in rice production around the world. Rice virus disease is characterized by uncontrolled virus replication and the activation of host responses that contribute to pathogenesis. Underlying these phenomena is the potent suppression of rice antiviral responses, particularly the RNA silencing pathway and plant hormone pathways, which play vital roles in antiviral immunity. Classical rice virus disease control strategies include chemotherapeutics and use of disease resistance rice varieties. Here, we summarize recent advances in understanding the mechanisms behind the immune evasion and rice viral pathogenesis. Based on these mechanistic insights, we discuss how to combine different strategies for maintaining the effectiveness of rice resistance to viruses, and propose theoretical basis for the next generation of virus-resistant rice plants. Keywords: Rice virus, Antiviral defense, Pathogenesis Background the biggest challenge is how to control rice virus dis- Rice is the most important crop in Asia and provides eases effectively and in an environmentally friendly staple food for half of the world’spopulation.Rice way. virus disease causes severe disease of rice in China Rice viruses are obligate intracellular parasites that and many other East Asia countries (Nicaise 2014). replicate, express viral proteins and establish infection Up to now, 17 rice virus diseases have been reported process in host cell. -

THE HUTTI GOLD MINES COMPANY LIMITED Place: Hutti
THE HUTTI GOLD MINES COMPANY LIMITED LIST OF CONTRACTORS WITH E.P.F REMMITTENCE No.of Remarks Employ Office of ees EPFO where Sl. Name of the Compliance PF Code engaged compliance No. contractor Up to through is being Contrac reported tor 1 Sri.Balavantappa, Class I Contractor, Work in KN/RCH/ 47446 14 EPFO Raichur Jul-2017 Ram Rahim Colony progress. Hutti-584115 2 M/s Shriram EPC Ltd., 4th Floor, Sigapi Achi Building 18/3 Rukmani GPRCH1352253 69 EPF Bengaluru Sept-2018 Lakshimipathi Salai Equare Chennai-600008. 3 M/s E-Infrastructure & Entertainment (India)Pvt Ltd, M.M Tower, B.Block BGBNG00800010 Bill cleared upto 168 Bangalore Jul-2018 No.01 jakkur plantation 00 Jul-2018 Road, Virupakshapura Bangalore - 560064 4 M/s Teknomin Construction Ltd., F-2, Rails VSR apartment, Opp VPS public 00390860 259 EPFO Raichur Nov-2018 School Mogalipuram, Vijayavada-520101 5 Sri Peersab S/o ImamSab, Labour Constructor, Work in Office Servant KN/RCR/ 47003 06 EPFO Raichur Oct-2018 progress. Quarter B-7 Po:Hutti Camp-584115 6 M/s Geetha Borewells Pvt Ltd ., EXISE RANGE Work order EPFO H.No 3-12-92/74 AAECG issued on Hyderabad Manvi, Taluk Mansoorbad 1771HSD001 24/08/2018 Hyderbad -500068. 7 M/s IC India Pvt Ltd Ro#565, 30th Main Road BGMRD10453270 kattariguppa BSK 3rd stage 32 Bangalore Nov-2018 Work Completed 00 Near Devegouda Petrol Bank Bangalore - 560083 8 M/s Adappa Chandappa Managoli H.No. 2-11-373 GBRCH00475640 Work in 11 Raichur Oct-2018 Near Laxmi Temple Raichur 00 progress Road Lingasugur - 584122 9 Sri K Mariyappa Work in Civil Works & Labour Supply GBRCH0047664000 39 EPFO RAICHUR Nov-2018 progress Contractor Hutti Camp 10 M/s Eranna Classs 1 GBRCH004727500 Raichur Work in Contractor Near FSI Talkies 0 21 Oct-2018 Progress Raichur Road, Manvi - 584123 11 Sri.Nilakantappa S/o Huchappa GBRCH163831300 PWD Class III Contractor 0 03 Raichur Work Stopped. -

Detection and Transmission of Rice Stunt Virus on Ciherang and Situ Bagendit Varieties
J.Helina HPT etTropika. al. Detection and Transmission of Rice StuntISSN: Virus 1411-7525 169 Vol. 18, No. 2, September 2018 E-ISSN: 2461-0399 Pages: 169–176 DOI : 10.23960/j.hptt.218169-176 DETECTION AND TRANSMISSION OF RICE STUNT VIRUS ON CIHERANG AND SITU BAGENDIT VARIETIES Selvi Helina1, Sri Sulandari1, Sedyo Hartono2, & Andi Trisyono2 1Department of Phytopathology, Faculty of Agriculture, Gadjah Mada University, Indonesia Jl. Flora No. 1 Bulaksumur Sleman Yogyakarta 55281 2Department of Plant Pests and Diseases, Faculty of Agriculture, Gadjah Mada University, Indonesia Jl. Flora No. 1 Bulaksumur Sleman Yogyakarta 55281 E-mail: [email protected] ABSTRACT Detection and Transmission of rice stunt virus on Ciherang and Situ Bagendit Varieties. The explosion of brown planthoppers recently has caused reduction of rice production in Indonesia. Brown planthoppers do not only act as pest, but also transmit Rice grassy stunt virus (RGSV) and Rice ragged stunt virus (RRSV). Detection of the existence of the two viruses in rice plants and vector insects is important to be done to ensure that the virus is infected with the vector. The aim of this research is to detect the existence of virus in varieties of Ciherang and Situ Bagendit as a result of transmission in the laboratory and to find out the ability of brown planthoppers to transmit stunt virus to both of the varieties. This research was compiled using Completely Randomized Design (CRD) with 4 treatments, namely healthy rice plants of Ciherang and Situ Bagendit varieties, Ciherang and Situ Bagendit varieties which were infested by brown planthoppers each with 5 repetitions. -

Rice Hoja Blanca: a Complex Plant–Virus–Vector Pathosystem F.J
CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 2010 5, No. 043 Review Rice hoja blanca: a complex plant–virus–vector pathosystem F.J. Morales* and P.R. Jennings Address: International Centre for Tropical Agriculture, AA 6713, Cali, Colombia. *Correspondence: F.J. Morales. E-mail: [email protected] Received: 30 April 2010 Accepted: 24 June 2010 doi: 10.1079/PAVSNNR20105043 The electronic version of this article is the definitive one. It is located here: http://www.cabi.org/cabreviews g CAB International 2009 (Online ISSN 1749-8848) Abstract Rice hoja blanca (RHB; white leaf) devastated rice (Oryza sativa) plantings in tropical America for half a century, before scientists could either identify its causal agent or understand the nature of its cyclical epidemics. The association of the planthopper Tagosodes orizicolus with RHB outbreaks, 20 years after its emergence in South America, suggested the existence of a viral pathogen. However, T. orizicolus could also cause severe direct feeding damage (hopperburn) to rice in the absence of hoja blanca, and breeders promptly realized that the genetic basis of resistance to these problems was different. Furthermore, it was observed that the causal agent of RHB could only be trans- mitted by a relatively low proportion of the individuals in any given population of T. orizicolus and that the pathogen was transovarially transmitted to the progeny of the planthopper vectors, affecting their normal biology. An international rice germplasm screening effort was initiated in the late 1950s to identify sources of resistance against RHB and the direct feeding damage caused by T. -

Lingasugur Bar Association : Lingasugur Taluk : Lingasugur District : Raichur
3/17/2018 KARNATAKA STATE BAR COUNCIL, OLD KGID BUILDING, BENGALURU VOTER LIST POLING BOOTH/PLACE OF VOTING : LINGASUGUR BAR ASSOCIATION : LINGASUGUR TALUK : LINGASUGUR DISTRICT : RAICHUR SL.NO. NAME SIGNATURE SHANKARGOWDA PAMPANGOUDA PATIL MYS/425/62 1 S/O PAMPANGOUDA PATIL ADVOCATE S.P. COMPLEX VIJAYA BANK ROAD LINGASUGUR RAICHUR 584122 MALLIKARJUN VENKATRAO JAGIRDAR MYS/174/68 2 S/O ADVOCATE BASAVESHWAR NAGAR : LINGASUGUR RAICHUR PATIL JAKKANAGOUDA BALANAGOUDA MYS/140/74 3 S/O BALANAGOUDA PATIL NEAR VIJAYA BANK NEAR GOVT HOSPITAL LINGASUGUR RAICHUR 584 122 KANAKAGIRI KASHIVISHWANATH VITHOBANNA KAR/321/77 4 S/O K. VITHOBANNA SHETTY VAIBHAVI NILAYA, HANUMAN CHOUK ,LINGASUGUR LINGASUGUR RAICHUR 584 122 1/28 3/17/2018 PATIL SHARANABASAVARAJ SANGANGOUDA KAR/155/79 5 S/O K SANGANAGOWDA LINGASUGUR LINGASUGUR RAICHUR 584122 AMARAPUR NAGAPPA KAR/427/79 S/O RAMAPPA 6 ADVOCATE RAGAVENDRA NILAYA,LAKSHMI NAGAR LINGASUGUR RAICHUR BALEGOUDA MALLIKARJUN AMARAPPA KAR/520/79 7 S/O AMARAPPA BALEGOWDA ADVOCATE HOUSING BOARD COLONY, M LINGASUGUR RAICHUR 584122 NAGALAPUR SHARANAPPA SHADAKSHARAPPA KAR/375/80 8 S/O SHADAKSHARAPPA OPP: COURT.SUB-JAIL AREA ,POST LINGASUGUR RAICHUR 584122 MAHABOOB ALI KAR/470/80 9 S/O RAJ MOHAMMED SAB ADVOCATE LINGASUGUR LINGASUGUR RAICHUR 584122 2/28 3/17/2018 IMADI AYYAPPA HANAMARADDEPPA KAR/474/82 10 S/O HANAMARADDEPPA KHB COLONY ,LINGASUGAR LINGASUGUR RAICHUR 584122 RAJASHEKHAR HONNAPPA KAR/73/83 11 S/O HONAPPA I.B. ROAD LINGASUGUR RAICHUR 584122 AMARESHWAR LINGARAJ SOMASHEKAR RAO KAR/30/86 12 S/O SOMASHEKAR RAO POST: MEDIKINHAL. LINGASUGUR RAICHUR SUNANDA SIDRAMAPPA BIRADAR KAR/872/90 D/O SIDRAMAPPA 13 W/O AMARASUNDAPPA. -

District Census Handbook, Raichur, Part II
CENSUS OF INDIA, 1951 HYDERABAD STATE District Census Handbook RAICHUR DISTl~ICT PART II Issued by BUREAU OF ECONOMICS AND STATISTICS FINANCE DEPARTMENT GOVERNMENT OF HYDERABAD PRICE Rs. 4 I. I I. I @ 0 I I I a: rn L&I IdJ .... U a::: Z >- c( &.41 IX :::::J c;m 0.: < a- w Q aiz LI.. Z 0 C 0 ::. Q .c( Q Will 1M III zZ et: 0 GIl :r -_,_,- to- t- U Col >->- -0'-0- 44 3I:i: IX a: ~ a:: ::. a w ti _, Ii; _, oc( -~-4a4<== > a at-a::a::. II: ..... e.. L&I Q In C a: o ....Co) a:: Q Z _,4 t- "Z III :? r o , '"" ,-. ~ I.:'; .. _ V ...._, ,. / .. l _.. I- 11.1 I en Col III -....IX ....% 1ft > c:a ED a: C :::::J 11.1 a. IX 4 < ~ Do. III -m a::: a. DISTRICT CONTENTS PAOB Frontiapkce MAP 0.1' RAICHUR DISTRICT Preface v Explanatory Note on Tables 1 List of Census Tracts-Raichur District 1. GENERAL POPULATION T"'BLES Table A-I-Area, Houses and Population 6 : Table A-II-Variation in Population during Fifty Years '8 Table A-Ill-Towns and Villages Classified by Population '10- , Table A-IV-Towns Classified by- Population with Variations since 1901 12' Table A-V-Towns arranged Territorially with Population by Livelihood Clasles 18 2. ECONOMIC TABLES Table B-I-Livelihood Classes and Sub-Classes 22 Table B-I1--Secondary Means of Livelihood 28 8. SOCIAL AND CULTURAL TABLES Table D-I-(i) Languages-Mother Tongue 82 Table D-I-(ii) Languages-Bi1ingmtli~m- - -,-, Table D-II-Religion Table D-III-Scheduled Castes and Scheduled Tribes Table D-VII-Literacy by Educational Standa'rds 4. -
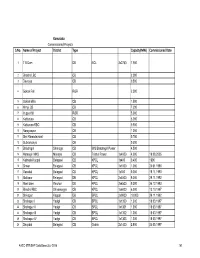
Karnataka Commissioned Projects S.No. Name of Project District Type Capacity(MW) Commissioned Date
Karnataka Commissioned Projects S.No. Name of Project District Type Capacity(MW) Commissioned Date 1 T B Dam DB NCL 3x2750 7.950 2 Bhadra LBC CB 2.000 3 Devraya CB 0.500 4 Gokak Fall ROR 2.500 5 Gokak Mills CB 1.500 6 Himpi CB CB 7.200 7 Iruppu fall ROR 5.000 8 Kattepura CB 5.000 9 Kattepura RBC CB 0.500 10 Narayanpur CB 1.200 11 Shri Ramadevaral CB 0.750 12 Subramanya CB 0.500 13 Bhadragiri Shimoga CB M/S Bhadragiri Power 4.500 14 Hemagiri MHS Mandya CB Trishul Power 1x4000 4.000 19.08.2005 15 Kalmala-Koppal Belagavi CB KPCL 1x400 0.400 1990 16 Sirwar Belagavi CB KPCL 1x1000 1.000 24.01.1990 17 Ganekal Belagavi CB KPCL 1x350 0.350 19.11.1993 18 Mallapur Belagavi DB KPCL 2x4500 9.000 29.11.1992 19 Mani dam Raichur DB KPCL 2x4500 9.000 24.12.1993 20 Bhadra RBC Shivamogga CB KPCL 1x6000 6.000 13.10.1997 21 Shivapur Koppal DB BPCL 2x9000 18.000 29.11.1992 22 Shahapur I Yadgir CB BPCL 1x1300 1.300 18.03.1997 23 Shahapur II Yadgir CB BPCL 1x1301 1.300 18.03.1997 24 Shahapur III Yadgir CB BPCL 1x1302 1.300 18.03.1997 25 Shahapur IV Yadgir CB BPCL 1x1303 1.300 18.03.1997 26 Dhupdal Belagavi CB Gokak 2x1400 2.800 04.05.1997 AHEC-IITR/SHP Data Base/July 2016 141 S.No. Name of Project District Type Capacity(MW) Commissioned Date 27 Anwari Shivamogga CB Dandeli Steel 2x750 1.500 04.05.1997 28 Chunchankatte Mysore ROR Graphite India 2x9000 18.000 13.10.1997 Karnataka State 29 Elaneer ROR Council for Science and 1x200 0.200 01.01.2005 Technology 30 Attihalla Mandya CB Yuken 1x350 0.350 03.07.1998 31 Shiva Mandya CB Cauvery 1x3000 3.000 10.09.1998 -

Hira Buddinni Gold Mine
Hira-Buddini Gold Mine project of M/s. Hutti Gold Mines Company Ltd for 21.825 Ha Mining lease area, at village-Hutti, Tehsil-Manvi, District-Raichur, Karnataka F.NO.J-11015/396/2005-IA-II(M) The total mine lease area of the project is 21.825 Ha Forest land is not involved out of 21.825 Ha lease area, 1.0 Ha is earmarked for overburden dump, 1.50 Ha for built up area/ infrastructure, 0.5 Ha for road, 4.0 Ha for green belt development and 14.825 Ha for other (undisturbed area). There is no National Park/Sanctuary/Biosphere reserve etc located within core and buffer zone. The targeted production capacity of the mine is 0.073 million tonnes per annum Mining is underground by semi-mechanised method Blasting is involved. COMPLIANCE REPORT Apr 2015 to Sept 2015 Project: Hira-Buddini Gold Project of M/s The Hutti Gold Mines Co Ltd., for 21.825 ha mining lease area at village Hira-Buddini, Tehsil – Manvi, District- Raichur, Karnataka – Environment Clarence. Ref: F.No.J-11015/396/2005-IA-II (M) Sl No. Specific Conditions Stipulated Compliance Status I Study report on subsidence and stage wise Reached-170 mts, massive rock, so far no subsidence has not development plan starting from 5th year of been occurred. operation till the end of the mine at an interval of 5 years should be submitted to the Ministry within six months. II The project authorities should take all safety All safety measures are being taken in accordance of measures in accordance of guidelines and guidelines and consultation of Director General Mines Safety consultation of Director General Mines Safety (DGMS) for development & Same will be continued for (DGMS) during development & mining operation. -

Upvc CASING PIPE DR.B.R.AMBEDKAR
DR.B.R.AMBEDKAR DEVELOPMENT CORPORATION LIMITED, BANGALORE M/S. SRI PANCHAMUKHI BOREWELLS, BOREWELLS DRILLED IN RAICHUR DIST UNDER IIBW SC FOR THE YEAR 2014-15 SL. NAME OF THE BENEFICIARY VILLAGE TALUK CONSTITUENCY NO. uPVC CASING PIPE 1 KANAKAPPA S/O GIRIYAMMA MACHANUR LINGASUGUR LINGASUGUR - 23 2 NANEPPA S/O KHOBANNA HOSAGUDDA LINGASUGUR LINGASUGUR - 24 3 SOMAPPA S/O GUNDAPPA MACHANUR THANDA LINGASUGUR LINGASUGUR - 28 4 BASAVARAJA S/O GUNDAPPA GOWDUR LINGASUGUR LINGASUGUR - 29 5 DURGAPPA S/O MADHYAPPA GOWDUR LINGASUGUR LINGASUGUR - 25 6 YALLAPPA S/O BALAPPA DEVARABUPUR LINGASUGUR LINGASUGUR - 32 7 YALLAMMA W/O MAREPPA BETADHUR MANVI MANVI - 11 8 JAGANAPPA S/O UMALAPPA NARABANDA THANDA MANVI MANVI - 27 9 RAMAPPA S/O HUCHAPPA HARAVI MANVI MANVI - 39 10 HULIGEPPA S/O AMARAPPA SAIDHAPURA MANVI MANVI - 31 11 RANGAPPA S/O THIMMAPPA KURUKUNDHA MANVI MANVI - 30 12 SHANKRAPPA S/O BHAGYANAYAK BETADHUR THANDA MANVI MANVI - 35 13 BHEEMANNA S/O DEVAPPA BHOVI KAVITHALA MANVI MANVI - 13 14 MEGHA S/O PURYA NEERAMANVI THANDA MANVI MANVI - 23 15 MUKKANNA S/O BANGYANAYAK NEERAMANVI THANDA MANVI MANVI - 24 16 DEVAPPA S/O RAGHAPPA MURKIGUDDA MANVI MANVI - 14 17 MAREMMA D/O AMARAMMA HIREKOTNEKAL MANVI MANVI - 01 18 HANUMANTHA S/O SABANNA MARATA MANVI MANVI - 08 19 BASAPPA S/O MUKAPPA MADLAPURA MANVI MANVI - 28 20 SHIVAPPA S/O HANUMAPPA KURUKUNDHA MANVI MANVI - 09 21 DHARMARAYA S/O DURGAVVA NEERAMANVI MANVI MANVI - 36 22 KARIYAPPA S/O HUSENI DHUMATHI SINDHANUR SINDHANUR - 09 23 HANUMANTHA S/O CHANDAPPA PAGADADINNI SINDHANUR SINDHANUR - 17 24 DURGAMMA W/O -

Rice Package
Rice INTEGRATED PEST MANAGEMENT INNOVATION LAB rice yaleclimateconnections.org package ice is an annual, self-pollinated, and semi-aquatic plant and belongs to the family Poaceae. Asian rice (Oryza sativa; subsp. japonica and indica), WHAT IS IPM? RAfrican rice (Oryza glaberrima), and wild rice (genus Zizania) are known to be consumed by humans. Oryza sativa subsp. indica was first domesticated Integrated pest management (IPM), an in India, whereas Oryza sativa subsp. japonica was domesticated in China. Rice environmentally-sound and economical is the most important food crop in the world and is a staple food across Asia approach to pest control, was developed and becoming important in Africa and Latin America. The traditional method of in response to pesticide misuse in cultivating rice is flooding the direct-seeded fields with or after transplanting the 1960s. Pesticide misuse has led to the young seedlings and is called irrigated rice production. Rice is also grown pesticide resistance among prevailing in the rainfed lowland, in mountains or plateaus, and the deep water. About pests, a resurgence of non-target pests, loss of biodiversity, and environmental 90 percent of rice production occurs in Asia. Although rice consumption and and human health hazards. demand are increasing around the globe, especially in Asia, stability in rice production in Asia depends on social and political stability. Climate change plays a major role in rice production in Asia. Irrigated rice area provides major production, but it is hard to increase irrigated rice area because of the WHAT ARE Lab (IPM IL) Management Innovation Pest Integrated problems of soil salinity, high cost of development, water scarcity, alternative IPM PACKAGES? and competing uses of water, and environmental concerns of the emission of greenhouse gases. -

RESEARCH on RICE VIRUS DISEASES in CHINA Xie Lian Hui*
4:5 RESEARCH ON RICE VIRUS DISEASES IN CHINA Xie Lian Hui* ABSTRACT As a result of investigations, a total of 11 virut' and ,·irus-like disease;, of rice, namely, yellow dwarf, dwarf, transitory yellowing, black-streaked dwarf, stripe, grassy stunt, bunchy stunt, ragged stunt, orange leaf, tungro and gall dwarf have been described. Among them, rhe causal agents of transitory yellowing and bunchy stunt were first identified and confirmed in China. The former occurred seriously in Taiwan, Yunnan, Guangxi, Guangdong. Fujian, Jiangxi, Hunan, Hubei. Zhejiang, Jiangsu, Shanghai, Anhui and Sichuan provinces, and the latter occurred only in some parts of Fujian, Jiangxi, Hunan and Guangdong provinces in China. As far as the distribution and the damage caused by these dise::ises arc concerned, transitory yellowing and dwarf are the most important. This report also deals with the advances and problems in the ecology, variety resistance and integrated control of rice virus diseases in China, and outiines some of the aspects which should be emphasized in the future. Introduction Virus diseases of rice were recorded long ago in China. However, it vvas not until the early 1960s that the diseases were comprehensively studied when transitory yellowing and black streaked dwarf occurred seriously in the south, the southwest and the east of China respectively. Since then, a series of spectacular results have been obtained in the investigations on disease incidence, etiology, ecology, variety resistance and integrated control. This report deals with the progress made in the research into rice virus diseases in China. Occurrence of the diseases Up to the present, a total of 16 virus and mycoplasma-like diseases of rice have been recorded in the world, 11 of which, namely, yellow dwarf, dwarf (RDV), transitory yellowing (RTYV), black-streaked dwarf (RBSDV), stripe (RSV), grassy stunt (RGSV), bunchy stunt (RBSV), ragged stunt (RRSV), orange leaf (ROLV), tungro (RTV) and gall dwarf (RGDV), have been observed in China. -

(Oryza Spp.) List of Descriptors
Descriptors for wild and cultivated Rice(Oryza spp.) List of Descriptors Allium (E,S) 2000 Peach * (E) 1985 Almond (revised) * (E) 1985 Pear * (E) 1983 Apple * (E) 1982 Pearl millet (E,F) 1993 Apricot * (E) 1984 Pepino (E) 2004 Avocado (E,S) 1995 Phaseolus acutifolius (E) 1985 Bambara groundnut (E,F) 2000 Phaseolus coccineus * (E) 1983 Banana (E,S,F) 1996 Phaseolus lunatus (P) 2001 Barley (E) 1994 Phaseolus vulgaris * (E,P) 1982 Beta (E) 1991 Pigeonpea (E) 1993 Black pepper (E,S) 1995 Pineapple (E) 1991 Brassica and Raphanus (E) 1990 Pistacia (excluding Pistacia vera) (E) 1998 Brassica campestris L. (E) 1987 Pistachio (E,F,A,R) 1997 Buckwheat (E) 1994 Plum * (E) 1985 Capsicum * (E,S) 1995 Potato variety * (E) 1985 Cardamom (E) 1994 Quinua * (S) 1981 Carrot (E,S,F) 1999 Rambutan (E) 2003 Cashew * (E) 1986 Rice * (E) 1980 Chenopodium pallidicaule (S) 2005 Rocket (E,I) 1999 Cherry * (E) 1985 Rye and Triticale * (E) 1985 Chickpea (E) 1993 Safflower * (E) 1983 Citrus (E,F,S) 1999 Sesame * (E) 2004 Coconut (E) 1992 Setaria italica and S. pumila (E) 1985 Coffee (E,S,F) 1996 Shea tree (E) 2006 Cotton * (Revised) (E) 1985 Sorghum (E,F) 1993 Cowpea * (E) 1983 Soyabean * (E,C) 1984 Cultivated potato * (E) 1977 Strawberry (E) 1986 Date palm (F) 2005 Sunflower * (E) 1985 Echinochloa millet * (E) 1983 Sweet potato (E,S,F) 1991 Eggplant (E,F) 1990 Taro (E,F,S) 1999 Faba bean * (E) 1985 Tea (E,S,F) 1997 Fig (E) 2003 Tomato (E, S, F) 1996 Finger millet * (E) 1985 Tropical fruit * (E) 1980 Forage grass * (E) 1985 Ulluco (S) 2003 Forage legumes * (E) 1984 Vigna aconitifolia and V.