Anthropogenic Impacts on Waituna Lagoon: Reconstructing The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Conversation with the Mayor Gary Tong
1 IN CONVERSATION WITH THE MAYOR GARY TONG through new technology (such as through our roading team’s use of drones). On a personal note, two things have stood have out this year; one of great sadness, the other a highlight. Sadly, we farewelled former Mayor Frana Cardno in April. She was a great role model and the reason I got into politics; a wonderful woman who will be sadly missed. Rest in peace, Frana. At the other end of the spectrum, in May I helped host His Mayor Gary Tong Royal Highness Prince Harry’s visit to Stewart Island. He’s a top bloke whose visit generated fantastic publicity for the Much like before crossing the road, island and Southland District. I’m sure our tourism industry at the end of each year I like to will see the benefi ts for some while yet. pause and look both ways. Just a few months ago the Southland Regional Development Strategy was launched. It gives direction for development of the region as a whole, with the primary focus on increasing our population. It tells us focusing on population growth will There’s a lot to look back on in 2015, and mean not only more people, it will provide economic growth, there’s plenty to come in 2016. Refl ecting on skilled workers, a better lifestyle, and improved health, the year that’s been, I realise just how much education and social services. We need to work together has happened in Southland District over the to achieve this; not just councils, but business, community, past year. -
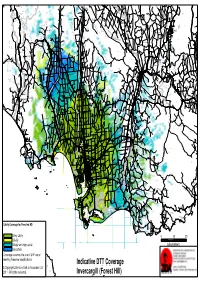
Indicative DTT Coverage Invercargill (Forest Hill)
Blackmount Caroline Balfour Waipounamu Kingston Crossing Greenvale Avondale Wendon Caroline Valley Glenure Kelso Riversdale Crossans Corner Dipton Waikaka Chatton North Beaumont Pyramid Tapanui Merino Downs Kaweku Koni Glenkenich Fleming Otama Mt Linton Rongahere Ohai Chatton East Birchwood Opio Chatton Maitland Waikoikoi Motumote Tua Mandeville Nightcaps Benmore Pomahaka Otahu Otamita Knapdale Rankleburn Eastern Bush Pukemutu Waikaka Valley Wharetoa Wairio Kauana Wreys Bush Dunearn Lill Burn Valley Feldwick Croydon Conical Hill Howe Benio Otapiri Gorge Woodlaw Centre Bush Otapiri Whiterigg South Hillend McNab Clifden Limehills Lora Gorge Croydon Bush Popotunoa Scotts Gap Gordon Otikerama Heenans Corner Pukerau Orawia Aparima Waipahi Upper Charlton Gore Merrivale Arthurton Heddon Bush South Gore Lady Barkly Alton Valley Pukemaori Bayswater Gore Saleyards Taumata Waikouro Waimumu Wairuna Raymonds Gap Hokonui Ashley Charlton Oreti Plains Kaiwera Gladfield Pikopiko Winton Browns Drummond Happy Valley Five Roads Otautau Ferndale Tuatapere Gap Road Waitane Clinton Te Tipua Otaraia Kuriwao Waiwera Papatotara Forest Hill Springhills Mataura Ringway Thomsons Crossing Glencoe Hedgehope Pebbly Hills Te Tua Lochiel Isla Bank Waikana Northope Forest Hill Te Waewae Fairfax Pourakino Valley Tuturau Otahuti Gropers Bush Tussock Creek Waiarikiki Wilsons Crossing Brydone Spar Bush Ermedale Ryal Bush Ota Creek Waihoaka Hazletts Taramoa Mabel Bush Flints Bush Grove Bush Mimihau Thornbury Oporo Branxholme Edendale Dacre Oware Orepuki Waimatuku Gummies Bush -

Section 6 Schedules 27 June 2001 Page 197
SECTION 6 SCHEDULES Southland District Plan Section 6 Schedules 27 June 2001 Page 197 SECTION 6: SCHEDULES SCHEDULE SUBJECT MATTER RELEVANT SECTION PAGE 6.1 Designations and Requirements 3.13 Public Works 199 6.2 Reserves 208 6.3 Rivers and Streams requiring Esplanade Mechanisms 3.7 Financial and Reserve 215 Requirements 6.4 Roading Hierarchy 3.2 Transportation 217 6.5 Design Vehicles 3.2 Transportation 221 6.6 Parking and Access Layouts 3.2 Transportation 213 6.7 Vehicle Parking Requirements 3.2 Transportation 227 6.8 Archaeological Sites 3.4 Heritage 228 6.9 Registered Historic Buildings, Places and Sites 3.4 Heritage 251 6.10 Local Historic Significance (Unregistered) 3.4 Heritage 253 6.11 Sites of Natural or Unique Significance 3.4 Heritage 254 6.12 Significant Tree and Bush Stands 3.4 Heritage 255 6.13 Significant Geological Sites and Landforms 3.4 Heritage 258 6.14 Significant Wetland and Wildlife Habitats 3.4 Heritage 274 6.15 Amalgamated with Schedule 6.14 277 6.16 Information Requirements for Resource Consent 2.2 The Planning Process 278 Applications 6.17 Guidelines for Signs 4.5 Urban Resource Area 281 6.18 Airport Approach Vectors 3.2 Transportation 283 6.19 Waterbody Speed Limits and Reserved Areas 3.5 Water 284 6.20 Reserve Development Programme 3.7 Financial and Reserve 286 Requirements 6.21 Railway Sight Lines 3.2 Transportation 287 6.22 Edendale Dairy Plant Development Concept Plan 288 6.23 Stewart Island Industrial Area Concept Plan 293 6.24 Wilding Trees Maps 295 6.25 Te Anau Residential Zone B 298 6.26 Eweburn Resource Area 301 Southland District Plan Section 6 Schedules 27 June 2001 Page 198 6.1 DESIGNATIONS AND REQUIREMENTS This Schedule cross references with Section 3.13 at Page 124 Desig. -

1 Phylogenetic Regionalization of Marine Plants Reveals Close Evolutionary Affinities Among Disjunct Temperate Assemblages Barna
Phylogenetic regionalization of marine plants reveals close evolutionary affinities among disjunct temperate assemblages Barnabas H. Darua,b,*, Ben G. Holtc, Jean-Philippe Lessardd,e, Kowiyou Yessoufouf and T. Jonathan Daviesg,h aDepartment of Organismic and Evolutionary Biology and Harvard University Herbaria, Harvard University, Cambridge, MA 02138, USA bDepartment of Plant Science, University of Pretoria, Private Bag X20, Hatfield 0028, Pretoria, South Africa cDepartment of Life Sciences, Imperial College London, Silwood Park Campus, Ascot SL5 7PY, United Kingdom dQuebec Centre for Biodiversity Science, Department of Biology, McGill University, Montreal, QC H3A 0G4, Canada eDepartment of Biology, Concordia University, Montreal, QC, H4B 1R6, Canada; fDepartment of Environmental Sciences, University of South Africa, Florida campus, Florida 1710, South Africa gDepartment of Biology, McGill University, Montreal, QC H3A 0G4, Canada hAfrican Centre for DNA Barcoding, University of Johannesburg, PO Box 524, Auckland Park, Johannesburg 2006, South Africa *Corresponding author Email: [email protected] (B.H. Daru) Running head: Phylogenetic regionalization of seagrasses 1 Abstract While our knowledge of species distributions and diversity in the terrestrial biosphere has increased sharply over the last decades, we lack equivalent knowledge of the marine world. Here, we use the phylogenetic tree of seagrasses along with their global distributions and a metric of phylogenetic beta diversity to generate a phylogenetically-based delimitation of marine phytoregions (phyloregions). We then evaluate their evolutionary affinities and explore environmental correlates of phylogenetic turnover between them. We identified 11 phyloregions based on the clustering of phylogenetic beta diversity values. Most phyloregions can be classified as either temperate or tropical, and even geographically disjunct temperate regions can harbor closely related species assemblages. -

Short Walks 2 up April 11
a selection of Southland s short walks contents pg For the location of each walk see the centre page map on page 17 and 18. Introduction 1 Information 2 Track Symbols 3 1 Mavora Lakes 5 2 Piano Flat 6 3 Glenure Allan Reserve 7 4 Waikaka Way Walkway 8 5 Croydon Bush, Dolamore Park Scenic Reserves 9,10 6 Dunsdale Reserve 11 7 Forest Hill Scenic Reserve 12 8 Kamahi/Edendale Scenic Reserve 13 9 Seaward Downs Scenic Reserve 13 10 Kingswood Bush Scenic Reserve 14 11 Borland Nature Walk 14 12 Tuatapere Scenic Reserve 15 13 Alex McKenzie Park and Arboretum 15 14 Roundhill 16 Location of walks map 17,18 15 Mores Scenic Reserve 19,20 16 Taramea Bay Walkway 20 17 Sandy Point Domain 21-23 18 Invercargill Estuary Walkway 24 19 Invercargill Parks & Gardens 25 20 Greenpoint Reserve 26 21 Bluff Hill/Motupohue 27,28 22 Waituna Viewing Shelter 29 23 Waipapa Point 30 24 Waipohatu Recreation Area 31 25 Slope Point 32 26 Waikawa 32 27 Curio Bay 33 Wildlife viewing 34 Walks further afield 35 For more information 36 introduction to short Southland s walking tracks short walks Short walking tracks combine healthy exercise with the enjoyment of beautiful places. They take between 15 minutes and 4 hours to complete Southland is renowned for challenging tracks that are generally well formed and maintained venture into wild and rugged landscapes. Yet many of can be walked in sensible leisure footwear the region's most attractive places can be enjoyed in a are usually accessible throughout the year more leisurely way – without the need for tramping boots are suitable for most ages and fitness levels or heavy packs. -

Evaluation of the Host Range of Hydrellia Lagarosiphon
Host range tests of Hydrellia lagarosiphon a candidate agent for Lagarosiphon major in New Zealand: assessing the risk to non-target species in New Zealand Jan-Robert Baars (University College Dublin), Quentin Paynter (Landcare Research New Zealand) Materials and Methods Selection of test plants A test plant list for New Zealand (henceforth NZ) (Table 1) was compiled using the centrifugal phylogenetic method (Wapshere, 1974) with amendments, as suggested by Briese and Walker (2002) and Briese (2003). Recent development in phylogenetics for the Hydrocharitaceae and its relation to taxonomically similar plant families were obtained by consulting the Angiosperm Phylogeny Website (Stevens, 2001 onwards; Fig. 1). Figure 1 Phylogeny of the Alismatales obtained from the Angiosperm Phylogeny Website: (Stevens, 2001 onwards) The most recent checklist of native NZ plants (De Lange and Rolfe, 2010) was examined to identify the NZ plant species that are most closely-related to lagarosiphon in order to compile a list of native plants for inclusion in host-range testing. Given that the aquatic lifestyle is a highly specialised one, relying totally on taxonomic position without due consideration of habitat has the potential to result in unsuitable test plants being included in a test list. For example, arthropod herbivores that feed on lagarosiphon are adapted to being submerged in freshwater and we can be sure that the marine eelgrass Zostera muelleri (Zosteriaceae) cannot be a suitable host for an insect herbivore because no insects have followed seagrasses into the ocean (Ollerton and McCollin, 1998). Zostera muelleri was, therefore, excluded from host- range testing. Wolffia australiana was also excluded from host-range testing as the tiny (0.3-1 mm long) platelets are far too small to be at risk of supporting the development of a leaf- mining fly. -

THE NEW ZEALAND GAZETTE [No
1692 THE NEW ZEALAND GAZETTE [No. 79 Grove Bush, Public School. Rotorua, Public School (principal). Haldane, Public Hall. Rotorua, Te Ngae Road, Mr. D. G. Osborne's Garage. Half-moon Bay, Stewart Island, Public School. Rotorua, Town Hall, Concert Chamber. Hawthorndale, East Road, Mission Church Hall. Ruatahuna, Native School. Hedgehope, Public School. Ruatoki North, Wilson's Store Shed. Houipapa, Houipapa Store. Taneatua, Hall. Kahuika, Public School. Te Kaha, Hall. Kapuka, Oteramika Hall. Te Teko, Native School. Kapuka South, Public School. Te Whaiti, Waikotikoti Dining-room. Kennington, Public School. Thornton, Hall. Lochiel, Public School. Toatoa, Public School. Longbush, Public School. Torere, Native School. Mabel Bush, Public Hall. Waimana, Public School. Maclennan, Public School. Wainui (Kutarere), Public School. Makarewa North, Public Hall. Waioeka (Opotiki), Hall. Makarewa Township, Public School. Waiohou, Native School. Mataura Island, Public School. Waiotahi, Settler's Hall. Menzies Ferry, Public School. Waiotapu, No.1 Camp, Forestry Hut. Mimihau, Public School. Wairata, School Building. Mokoreta, Public School. Whakarewarewa, Forestry Training Centre,. Lecture Room. Mokotua, Tennis Club's Hall. Whakarewarewa, Waipa Mill, Hall. Morton Mains (Siding), Public School. Whakatane, Borough Council Chambers. Myross Bush, Public School. Whakatane, County Council Chambers. Niagara, Public School. Whakatane Paper Mills, Recreation-room. Oreti, Sunday School Hall. Woodlands (Opotiki), Public School. Otahuti, Public School. Otara, Public School. Brooklyn Electoral District- Otatara, Public School. Oteramika Road, Sunday School Hall. Adelaide Road, Empty Shop at No. 125. Pine Bush, Public School. Adelaide Road, St. James's Church Hall. Progress Valley, Public School. Aro Street, St. Mary of the Angels School. Quarry Hills, Public School. Brooklyn, Ohiro Road, Baptist Church Hall. -

Year 12 Biology Camp in Dunedin Newsletter
Year 12 Biology Camp in Dunedin Newsletter 7 Newsletter 17 May 2013 Principal: Grant Dick [email protected] www.csc.school.nz Phone 03 236 7646 - Fax 03 236 7645 - Grange Street, P O Box 94, Winton Editorial A very warm welcome back to our school community to Term 2 2013, and a big thank you to everybody for such an overwhelming welcome for me as I settle into this new position as Principal at Central Southland College. Appreciation must be given to Mrs Summers, and in fact all the staff, for leading this school over the past little while, and enabling me to walk into an environment that is providing a solid, stimulating and exciting learning experience for our students to thrive in. At my first assembly I spoke about my first impression of this school, and that was watching a large group of our students who had bussed into town to support and cheer on our rugby team. The students were well presented, encouraging of each other and represented our school values extremely well. This is so important wherever we are; home, school or in the community, when a large group of our students can display this when they don’t realise they are ‘being watched’ tells me these values are well ingrained. I am really looking forward to getting to know our school community, please don’t hesitate to make yourself known to me whether at a meeting, on the sporting side line, or at an event. I would like to wish our stage challenge crew all the best as they head on stage tonight. -

Natural History of the Coorong
Natural History of the Coorong, Lower Lakes, and Murray Mouth Region (yarluwar-ruwe) This book is available as a free fully searchable ebook from www.adelaide.edu.au/press Occasional publications of the Royal Society of South Australia Inc. Ideas & Endeavours: a History of the Natural Sciences in South Australia, published 1986. Natural History of the Adelaide Region, published 1976, reprinted 1988. Natural History of Eyre Peninsula, published 1985. Natural History of the Flinders Ranges, published 1996. Natural History of Kangaroo Island, second edition, published 2002. Natural History of the North East Deserts, published 1990. Natural History of the South East, published 1983, reprinted 1995. Natural History of Gulf St Vincent, published 2008. Natural History of Riverland and Murraylands, published 2009. Natural History of Spencer Gulf, published 2014. Natural History of the Coorong, Lower Lakes, and Murray Mouth Region (yarluwar-ruwe) Editors Luke Mosley, Qifeng Ye, Scoresby Shepherd, Steve Hemming, Rob Fitzpatrick Royal Society of South Australia Inc. Published in Adelaide by University of Adelaide Press Barr Smith Library The University of Adelaide South Australia 5005 [email protected] www.adelaide.edu.au/press on behalf of the Royal Society of South Australia Inc. © 2018 Royal Society of South Australia. This work is licenced under the Creative Commons Attribution-NonCommercial- NoDerivatives 4.0 International (CC BY-NC-ND 4.0) License. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0 or send a letter to Creative Commons, 444 Castro Street, Suite 900, Mountain View, California, 94041, USA. This licence allows for the copying, distribution, display and performance of this work for non-commercial purposes providing the work is clearly attributed to the copyright holders. -

Edendale Community Response Plan 2018
Southland has NO Civil Defence sirens (fire brigade sirens are not used as warnings for a Civil Defence emergency) Edendale Community Response Plan 2018 If you’d like to become part of the Edendale Community Response Group Please email [email protected] Find more information on how you can be prepared for an emergency www.cdsouthland.nz Community Response Planning In the event of an emergency, communities may need to support themselves for up to 10 days before assistance arrives. The more prepared a community is, the more likely it is that the community will be able to look after themselves and others. This plan contains a short demographic description of Edendale, information about key hazards and risks, information about Community Emergency Hubs where the community can gather, and important contact information to help the community respond effectively. Members of the Edendale Community Response Group have developed the information contained in this plan and will be Emergency Management Southland’s first points of community contact in an emergency. Demographic Details • Edendale is contained within the Southland District Council area; • Edendale has a population of approximately 555 people and the population of the wider area is approximately 2,400 people; • State Highway 1 goes through Edendale; • the KiwiRail main trunk line for the South Island passes through Edendale; • The town has a milk processing plant operated by Fonterra; • The broad geographic area for the Edendale Community Response Plan includes Brydone, Seaward Downs, Gorge Road, Mokotua, Rimu, Waimatua, Mabel Bush, Woodlands, Dacre and Te Tipua; • The town has a fire service, primary school, and an early learning childcare facility. -

ASBS Newsletter I Gave an Overview of Outside Our Sector), and of the Importance and Taxonomy Australia and Its Role and Governance
Newsletter No. 177 December 2018 Price: $5.00 AUSTRALASIAN SYSTEMATIC BOTANY SOCIETY INCORPORATED Council President Vice President Darren Crayn Daniel Murphy Australian Tropical Herbarium (CNS) Royal Botanic Gardens Victoria James Cook University, Cairns Campus Birdwood Avenue PO Box 6811, Cairns Qld 4870 Melbourne, Vic. 3004 Australia Australia Tel: (+617)/(07) 4232 1859 Tel: (+613)/(03) 9252 2377 Email: [email protected] Email: [email protected] Secretary Treasurer Jennifer Tate Matt Renner Institute of Fundamental Sciences Royal Botanic Garden Sydney Massey University Mrs Macquaries Road Private Bag 11222, Palmerston North 4442 Sydney NSW 2000 New Zealand Australia Tel: (+646)/(6) 356- 099 ext. 84718 Tel: (+61)/(0) 415 343 508 Email: [email protected] Email: [email protected] Councillor Councillor Ryonen Butcher Heidi Meudt Western Australian Herbarium Museum of New Zealand Te Papa Tongarewa Locked Bag 104 PO Box 467, Cable St Bentley Delivery Centre WA 6983 Wellington 6140, New Zealand Australia Tel: (+644)/(4) 381 7127 Tel: (+618)/(08) 9219 9136 Email: [email protected] Email: [email protected] Other constitutional bodies Hansjörg Eichler Research Committee Affiliate Society David Glenny Papua New Guinea Botanical Society Sarah Mathews Heidi Meudt Joanne Birch Advisory Standing Committees Katharina Nargar Financial Murray Henwood Patrick Brownsey Chair: Dan Murphy, Vice President, ex officio David Cantrill Grant application closing dates Bob Hill Hansjörg Eichler Research Fund: th th Ad -

Phylogenetics and Molecular Evolution of Alismatales Based on Whole Plastid Genomes
PHYLOGENETICS AND MOLECULAR EVOLUTION OF ALISMATALES BASED ON WHOLE PLASTID GENOMES by Thomas Gregory Ross B.Sc. The University of British Columbia, 2011 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIRMENTS FOR THE DEGREE OF MASTER OF SCIENCE in The Faculty of Graduate and Postdoctoral Studies (Botany) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) November 2014 © Thomas Gregory Ross, 2014 ABSTRACT The order Alismatales is a mostly aquatic group of monocots that displays substantial morphological and life history diversity, including the seagrasses, the only land plants that have re-colonized marine environments. Past phylogenetic studies of the order have either considered a single gene with dense taxonomic sampling, or several genes with thinner sampling. Despite substantial progress based on these studies, multiple phylogenetic uncertainties still remain concerning higher-order phylogenetic relationships. To address these issues, I completed a near- genus level sampling of the core alismatid families and the phylogenetically isolated family Tofieldiaceae, adding these new data to published sequences of Araceae and other monocots, eudicots and ANITA-grade angiosperms. I recovered whole plastid genomes (plastid gene sets representing up to 83 genes per taxa) and analyzed them using maximum likelihood and parsimony approaches. I recovered a well supported phylogenetic backbone for most of the order, with all families supported as monophyletic, and with strong support for most inter- and intrafamilial relationships. A major exception is the relative arrangement of Araceae, core alismatids and Tofieldiaceae; although most analyses recovered Tofieldiaceae as the sister-group of the rest of the order, this result was not well supported. Different partitioning schemes used in the likelihood analyses had little effect on patterns of clade support across the order, and the parsimony and likelihood results were generally highly congruent.