Bark Anatomy of an Early Carboniferous Tree from Australia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Comparative Study of the Primary Vascular System Of
Amer. J. Bot. 55(4): 464-472. 1!16'>. A COMPARATIVE STUDY OF THE PRIMARY VASCULAR SYSTE~1 OF CONIFERS. III. STELAR EVOLUTION IN GYMNOSPERMS 1 KADAMBARI K. NAMBOODIRI2 AND CHARLES B. BECK Department of Botany, University of Michigan, Ann Arbor ABST RAe T This paper includes a survey of the nature of the primary vascular system in a large number of extinct gymnosperms and progymnosperms. The vascular system of a majority of these plants resembles closely that of living conifers, being characterized, except in the most primitive forms which are protostelic, by a eustele consisting of axial sympodial bundles from which leaf traces diverge. The vascular supply to a leaf originates as a single trace with very few exceptions. It is proposed that the eustele in the gyrr.nosperms has evolved directly from the protostele by gradual medullation and concurrent separation of the peripheral conducting tissue into longitudinal sympodial bundles from which traces diverge radially. A subsequent modification results in divergence of traces in a tangential plane, The closed vascular system of conifers with opposite and whorled phyllotaxis, in which the vascular supply to a leaf originates as two traces which subsequently fuse, is considered to be derived from the open sympodial system characteristic of most gymnosperms. This hypothesis of stelar evolution is at variance with that of Jeffrey which suggests that the eustele of seed plants is derived by the lengthening and overlapping of leaf gaps in a siphonostele followed by further reduction in the resultant vascular bundles. This study suggests strongly that the "leaf gap" of conifers and other extant gymnosperms is not homologous with that of siphonostelic ferns and strengthens the validity of the view that Pterop sida is an unnatural group. -

Human Interaction with Technology for Working, Communicating, and Learning: Advancements
An Excellent Addition to Your Library! Released: December 2011 Human Interaction with Technology for Working, Communicating, and Learning: Advancements Anabela Mesquita (ISCAP/IPP and Algoritmi Centre, University of Minho, Portugal) The way humans interact with technology is undergoing a tremendous change. It is hard to imagine the lives we live today without the benefits of technology that we take for granted. Applying research in computer science, engineer- ing, and information systems to non-technical descriptions of technology, such as human interaction, has shaped and continues to shape our lives. Human Interaction with Technology for Working, Communicating, and Learning: Advancements provides a framework for conceptual, theoretical, and applied research in regards to the relationship between technology and humans. This book is unique in the sense that it does not only cover technology, but also science, research, and the relationship between these fields and individuals’ experience. This book is a must have for anyone interested in this research area, as it provides a voice for all users and a look into our future. Topics Covered: • Anthropological Consequences • Philosophy of Technology • Experiential Learning • Responsibility of Artificial Agents • Influence of Gender on Technology • Technological Risks and Their Human Basis • Knowledge Management • Technology Assessments • Perceptions and Conceptualizations • Technology Ethics of Technology • Phenomenology of E-Government ISBN: 9781613504659; © 2012; 421 pp. Print: US $175.00 | Perpetual: US $265.00 | Print + Perpetual: US $350.00 Market: This premier publication is essential for all academic and research library reference collections. It is a crucial tool for academicians, researchers, and practitioners and is ideal for classroom use. Anabela Mesquita is a professor at the Institute of Administration and Accountancy (ISCAP)/Polytechnic School of Porto (IPP), Portugal. -

Heterospory: the Most Iterative Key Innovation in the Evolutionary History of the Plant Kingdom
Biol. Rej\ (1994). 69, l>p. 345-417 345 Printeii in GrenI Britain HETEROSPORY: THE MOST ITERATIVE KEY INNOVATION IN THE EVOLUTIONARY HISTORY OF THE PLANT KINGDOM BY RICHARD M. BATEMAN' AND WILLIAM A. DiMlCHELE' ' Departments of Earth and Plant Sciences, Oxford University, Parks Road, Oxford OXi 3P/?, U.K. {Present addresses: Royal Botanic Garden Edinburiih, Inverleith Rojv, Edinburgh, EIIT, SLR ; Department of Geology, Royal Museum of Scotland, Chambers Street, Edinburgh EHi ijfF) '" Department of Paleohiology, National Museum of Natural History, Smithsonian Institution, Washington, DC^zo^bo, U.S.A. CONTENTS I. Introduction: the nature of hf^terospon' ......... 345 U. Generalized life history of a homosporous polysporangiophyle: the basis for evolutionary excursions into hetcrospory ............ 348 III, Detection of hcterospory in fossils. .......... 352 (1) The need to extrapolate from sporophyte to gametophyte ..... 352 (2) Spatial criteria and the physiological control of heterospory ..... 351; IV. Iterative evolution of heterospory ........... ^dj V. Inter-cladc comparison of levels of heterospory 374 (1) Zosterophyllopsida 374 (2) Lycopsida 374 (3) Sphenopsida . 377 (4) PtiTopsida 378 (5) f^rogymnospermopsida ............ 380 (6) Gymnospermopsida (including Angiospermales) . 384 (7) Summary: patterns of character acquisition ....... 386 VI. Physiological control of hetcrosporic phenomena ........ 390 VII. How the sporophyte progressively gained control over the gametophyte: a 'just-so' story 391 (1) Introduction: evolutionary antagonism between sporophyte and gametophyte 391 (2) Homosporous systems ............ 394 (3) Heterosporous systems ............ 39(1 (4) Total sporophytic control: seed habit 401 VIII. Summary .... ... 404 IX. .•Acknowledgements 407 X. References 407 I. I.NIRODUCTION: THE NATURE OF HETEROSPORY 'Heterospory' sensu lato has long been one of the most popular re\ie\v topics in organismal botany. -
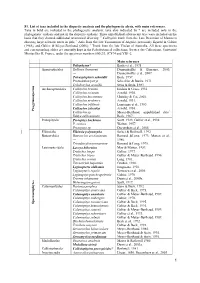
S1. List of Taxa Included in the Disparity Analysis and the Phylogenetic Alysis, with Main References
S1. List of taxa included in the disparity analysis and the phylogenetic alysis, with main references. Taxa in bold are included in the phylogenetic analysis; taxa also indicated by * are included only in the phylogenetic analysis and not in the disparity analysis. Three unpublished arborescent taxa were included on the basis that they showed additional anatomical diversity. 1 Callixylon trunk from the Late Devonian of Marrocco showing large sclerotic nests in pith; 2 Axis from the late Tournaisian of Algeria, previously figured in Galtier (1988), and Galtier & Meyer-Berthaud (2006); 3 Trunk from the late Viséan of Australia. All these specimens and corresponding slides are currently kept in the Paleobotanical collections, Service des Collections, Université Montpellier II, France, under the specimen numbers 600/2/3, JC874 and YB1-2. Main reference Psilophyton* Banks et al., 1975 Aneurophytales Rellimia thomsonii Dannenhoffer & Bonamo, 2003; --- Dannenhoffer et al., 2007. Tetraxylopteris schmidtii Beck, 1957. Proteokalon petryi Scheckler & Banks, 1971. Triloboxylon arnoldii Stein & Beck, 1983. s m Archaeopteridales Callixylon brownii Hoskin & Cross, 1951. r e Callixylon erianum Arnold, 1930. p s o Callixylon huronensis Chitaley & Cai, 2001. n Callixylon newberry Arnold, 1931. m y g Callixylon trifilievii Lemoigne et al., 1983. o r Callixylon zalesskyi Arnold, 1930. P Callixylon sp. Meyer-Berthaud, unpublished data1. Eddya sullivanensis Beck, 1967. Protopityales Protopitys buchiana Scott, 1923; Galtier et al., 1998. P. scotica Walton, 1957. Protopitys sp. Decombeix et al., 2005. Elkinsiales Elkinsia polymorpha Serbet & Rothwell, 1992. Buteoxylales Buteoxylon gordonianum Barnard &Long, 1973; Matten et al., --- 1980. Triradioxylon primaevum Barnard & Long, 1975. Lyginopteridales Laceya hibernica May & Matten, 1983. Tristichia longii Galtier, 1977. -

Structure, Development and Reproduction in Flowering Plants
Structure, Development and Reproduction in Flowering Plants Body Plan and Diversity in Form S.V.S Chauhan Professor Department of Botany B.R. Ambedkar University Khandari Campus Agra – 282002 [email protected] 1 Body Plan and Diversity in Form Every living organism has a fixed form and it is because of this reason that we are able to distinguish most of them just due to their external structure. Study of external morphology or external appearance of higher plants is necessary to describe the plants in an accurate fashion and to distinguish between almost similar looking plants. Therefore, the plants are identified by their morphological characters. Variation in plants is found not only in external forms but also in their anatomical characters which are represented by different types of tissue systems . Morphology along with anatomy constitute the base of studying pattern of life forms. Life Span of Plants On the basis of life span, plants are of three types: annuals, biennials and perennials. a) Annuals: These plants complete their life-cycle in a single growing season which varies from a few weeks to a few months. They pass the unfavourable period in the form of seeds. Examples are wheat, pea and sunflower, etc. b) Biennials: These plants complete their life-cycle in two growing seasons. In the first season; they grow only vegetatively and store food generally in the roots. In the second season, these plants grow at the expense of the stored food and form the flowering shoot bearing flowers, fruits and seeds. Then the plants die. radish, turnip, cabbage, etc. -

Second Call for Papers
SECOND CALL FOR PAPERS 3rd FRENCH CONFERENCE ON SOCIAL AND ENVIRONMENTAL ACCOUNTING RESEARCH (3rd CSEAR France Conference) June 11-12, 2015 ESSEC Business School, France The Centre for Social and Environmental Accounting Research (CSEAR) has held an annual conference in the UK (often referred to as the Summer School) for more than two decades, as well as in other parts of the world for several years in Europe, Australia, New Zealand, and in both North and South Americas. Similar to other CSEAR conferences, the 3rd CSEAR France Conference will be a deliberately informal discussion forum gathering researchers, teachers, students and practitioners at all levels of experience to further enhance research in new instruments, policies and strategies related to social and environmental accounting in the very widest sense. The spirit of the conference is interdisciplinary and focuses on a high level of interaction, discussion and debate in a friendly and supportive atmosphere. As such, research papers from perspectives beyond accounting and management control such as organizational theory, marketing, finance, strategy, etc., are welcome. The conference will provide participants an opportunity to present their research projects, ranging from discussing preliminary findings to full papers. Submissions are welcome in either French or English. For this 3rd CSEAR France conference, we are pleased to welcome Professor Brendan O’Dwyer, Professor of Accounting at the University of Amsterdam, as our plenary speaker. For the practitioner forum, we are equally pleased to welcome Adam Koniuszewski, Chief Operating Officer at Green Cross International. There will also be a Special Issue of Advances in Environmental Accounting and Management on the “Current Developments in Social and Environmental Accounting” (a call for papers has been issued and is available on the conference website). -

Earliest Record of Megaphylls and Leafy Structures, and Their Initial Diversification
Review Geology August 2013 Vol.58 No.23: 27842793 doi: 10.1007/s11434-013-5799-x Earliest record of megaphylls and leafy structures, and their initial diversification HAO ShouGang* & XUE JinZhuang Key Laboratory of Orogenic Belts and Crustal Evolution, School of Earth and Space Sciences, Peking University, Beijing 100871, China Received January 14, 2013; accepted February 26, 2013; published online April 10, 2013 Evolutionary changes in the structure of leaves have had far-reaching effects on the anatomy and physiology of vascular plants, resulting in morphological diversity and species expansion. People have long been interested in the question of the nature of the morphology of early leaves and how they were attained. At least five lineages of euphyllophytes can be recognized among the Early Devonian fossil plants (Pragian age, ca. 410 Ma ago) of South China. Their different leaf precursors or “branch-leaf com- plexes” are believed to foreshadow true megaphylls with different venation patterns and configurations, indicating that multiple origins of megaphylls had occurred by the Early Devonian, much earlier than has previously been recognized. In addition to megaphylls in euphyllophytes, the laminate leaf-like appendages (sporophylls or bracts) occurred independently in several dis- tantly related Early Devonian plant lineages, probably as a response to ecological factors such as high atmospheric CO2 concen- trations. This is a typical example of convergent evolution in early plants. Early Devonian, euphyllophyte, megaphyll, leaf-like appendage, branch-leaf complex Citation: Hao S G, Xue J Z. Earliest record of megaphylls and leafy structures, and their initial diversification. Chin Sci Bull, 2013, 58: 27842793, doi: 10.1007/s11434- 013-5799-x The origin and evolution of leaves in vascular plants was phology and evolutionary diversification of early leaves of one of the most important evolutionary events affecting the basal euphyllophytes remain enigmatic. -

Dr. Sahanaj Jamil Associate Professor of Botany M.L.S.M. College, Darbhanga
Subject BOTANY Paper No V Paper Code BOT521 Topic Taxonomy and Diversity of Seed Plant: Gymnosperms & Angiosperms Dr. Sahanaj Jamil Associate Professor of Botany M.L.S.M. College, Darbhanga BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants UNIT- I BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants Classification of Gymnosperms. # Robert Brown (1827) for the first time recognized Gymnosperm as a group distinct from angiosperm due to the presence of naked ovules. BENTHAM and HOOKSER (1862-1883) consider them equivalent to dicotyledons and monocotyledons and placed between these two groups of angiosperm. They recognized three classes of gymnosperm, Cyacadaceae, coniferac and gnetaceae. Later ENGLER (1889) created a group Gnikgoales to accommodate the genus giankgo. Van Tieghem (1898) treated Gymnosperm as one of the two subdivision of spermatophyte. To accommodate the fossil members three more classes- Pteridospermae, Cordaitales, and Bennettitales where created. Coulter and chamberlain (1919), Engler and Prantl (1926), Rendle (1926) and other considered Gymnosperm as a division of spermatophyta, Phanerogamia or Embryoptyta and they further divided them into seven orders: - i) Cycadofilicales ii) Cycadales iii) Bennettitales iv) Ginkgoales v) Coniferales vi) Corditales vii) Gnetales On the basis of wood structure steward (1919) divided Gymnosperm into two classes: - i) Manoxylic ii) Pycnoxylic The various classification of Gymnosperm proposed by various workers are as follows: - i) Sahni (1920): - He recognized two sub-divison in gymnosperm: - a) Phylospermae b) Stachyospermae BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants ii) Classification proposed by chamber lain (1934): - He divided Gymnosperm into two divisions: - a) Cycadophyta b) Coniterophyta iii) Classification proposed by Tippo (1942):- He considered Gymnosperm as a class of the sub- phylum pteropsida and divided them into two sub classes:- a) Cycadophyta b) Coniferophyta iv) D. -

IEEE IRI 2013 Main Conference Program
IEEE IRI 2013 Detailed Program, August 14-16, 2013, Sofitel Hotel, San Francisco, USA IEEE IRI 2013 Main Conference Program August 14, 2013, Wednesday (Day 1) 8:00 am - 5:00 pm Conference Registration (Conference Registration Desk at Ballroom Foyer) Informal Meet & Greet - Breakfast 7:30-8:15am (Veranda) Welcome, Conference Opening Remarks 8:15-8:30am (Bordeaux) KEYNOTE 1: Professor Lotfi A. Zadeh, UC Berkeley, CA, USA 8:30-9:30am Toward a Restriction-Centered Theory of Truth and Meaning (Bordeaux) 9:30–9:50am Break (Ballroom Foyer) 9:50am- Session A1 11:50am Session A11 (Grand Salon) Information Security & Privacy Chair: Du Zhang California State University, Sacramento, USA FIEP: An Initial Design of A Firewall Information Exchange Protocol 20 Sandeep Reddy Pedditi, Du Zhang and Chung-E Wang (Application Paper) California State University, Sacramento, USA Predicting Susceptibility to Social Bots on Twitter (1) (1) (1) (2) 33 Randall Wald , Taghi M. Khoshgoftaar , Amri Napolitano and Chris Sumner (1) (Regular Research Florida Atlantic University, USA paper) (2) Online Privacy Foundation, USA Simulation-Based Validation for Smart Grid Environments 85 Wonkyu Han, Michael Mabey and Gail-Joon Ahn (Regular Research paper) Arizona State University, USA A Moving Target Defense Approach for Protecting Resource-Constrained Distributed Devices 146 Valentina Casola (1) , Alessandra De Benedictis (1) and Massimiliano Albanese (2) (Regular Research (1) paper) University of Naples Federico II, Italy (2) George Mason University, USA Session A12 (Blue -

Bibliometric Study in Support of Norway's Strategy for International Research Collaboration
Bibliometric Study in Support of Norway's Strategy for International Research Collaboration Final report Bibliometric Study in Support of Norway's Strategy for International Research Collaboration Final report © The Research Council of Norway 2014 The Research Council of Norway P.O.Box 2700 St. Hanshaugen N–0131 OSLO Telephone: +47 22 03 70 00 Telefax: +47 22 03 70 01 [email protected] www.rcn.no/english The report can be ordered at: www.forskningsradet.no/publikasjoner or green number telefax: +47 800 83 001 Design cover: Design et cetera AS Printing: 07 Gruppen/The Research Council of Norway Number of copies: 150 Oslo, March 2014 ISBN 978-82-12-03310-8 (print) ISBN 978-82-12-03311-5 (pdf) Bibliometric Study in Support of Norway’s Strategy for International Research Collaboration Final Report March 14, 2014 Authors Alexandre Beaudet David Campbell Grégoire Côté Stefanie Haustein Christian Lefebvre Guillaume Roberge Other contributors Philippe Deschamps Leroy Fife Isabelle Labrosse Rémi Lavoie Aurore Nicol Bastien St-Louis Lalonde Matthieu Voorons Contact information Grégoire Côté, Vice-President, Bibliometrics [email protected] Brussels | Montreal | Washington Bibliometric Study in Support of Norway’s Strategy for Final Report International Research Collaboration Contents Contents .................................................................................................................. i Tables ................................................................................................................... iii -

Retallack 2021 Coal Balls
Palaeogeography, Palaeoclimatology, Palaeoecology 564 (2021) 110185 Contents lists available at ScienceDirect Palaeogeography, Palaeoclimatology, Palaeoecology journal homepage: www.elsevier.com/locate/palaeo Modern analogs reveal the origin of Carboniferous coal balls Gregory Retallack * Department of Earth Science, University of Oregon, Eugene, Oregon 97403-1272, USA ARTICLE INFO ABSTRACT Keywords: Coal balls are calcareous peats with cellular permineralization invaluable for understanding the anatomy of Coal ball Pennsylvanian and Permian fossil plants. Two distinct kinds of coal balls are here recognized in both Holocene Histosol and Pennsylvanian calcareous Histosols. Respirogenic calcite coal balls have arrays of calcite δ18O and δ13C like Carbon isotopes those of desert soil calcic horizons reflecting isotopic composition of CO2 gas from an aerobic microbiome. Permineralization Methanogenic calcite coal balls in contrast have invariant δ18O for a range of δ13C, and formed with anaerobic microbiomes in soil solutions with bicarbonate formed by methane oxidation and sugar fermentation. Respiro genic coal balls are described from Holocene peats in Eight Mile Creek South Australia, and noted from Carboniferous coals near Penistone, Yorkshire. Methanogenic coal balls are described from Carboniferous coals at Berryville (Illinois) and Steubenville (Ohio), Paleocene lignites of Sutton (Alaska), Eocene lignites of Axel Heiberg Island (Nunavut), Pleistocene peats of Konya (Turkey), and Holocene peats of Gramigne di Bando (Italy). Soils and paleosols with coal balls are neither common nor extinct, but were formed by two distinct soil microbiomes. 1. Introduction and Royer, 2019). Although best known from Euramerican coal mea sures of Pennsylvanian age (Greb et al., 1999; Raymond et al., 2012, Coal balls were best defined by Seward (1895, p. -

Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation
i1 Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation William A. DiMichele, William E. Stein, and Richard M. Bateman DiMichele, W.A., Stein, W.E., and Bateman, R.M. 2001. Ecological sorting of vascular plant classes during the Paleozoic evolutionary radiation. In: W.D. Allmon and D.J. Bottjer, eds. Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia University Press, New York. pp. 285-335 THE DISTINCTIVE BODY PLANS of vascular plants (lycopsids, ferns, sphenopsids, seed plants), corresponding roughly to traditional Linnean classes, originated in a radiation that began in the late Middle Devonian and ended in the Early Carboniferous. This relatively brief radiation followed a long period in the Silurian and Early Devonian during wrhich morphological complexity accrued slowly and preceded evolutionary diversifications con- fined within major body-plan themes during the Carboniferous. During the Middle Devonian-Early Carboniferous morphological radiation, the major class-level clades also became differentiated ecologically: Lycopsids were cen- tered in wetlands, seed plants in terra firma environments, sphenopsids in aggradational habitats, and ferns in disturbed environments. The strong con- gruence of phylogenetic pattern, morphological differentiation, and clade- level ecological distributions characterizes plant ecological and evolutionary dynamics throughout much of the late Paleozoic. In this study, we explore the phylogenetic relationships and realized ecomorphospace of reconstructed whole plants (or composite whole plants), representing each of the major body-plan clades, and examine the degree of overlap of these patterns with each other and with patterns of environmental distribution. We conclude that 285 286 EVOLUTIONARY PALEOECOLOGY ecological incumbency was a major factor circumscribing and channeling the course of early diversification events: events that profoundly affected the structure and composition of modern plant communities.