Malus 'Adirondack'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Method for Producing Methacrylic Acid And/Or Ester Thereof
(19) TZZ _T (11) EP 2 894 224 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: 15.07.2015 Bulletin 2015/29 C12P 7/62 (2006.01) C12N 15/09 (2006.01) (21) Application number: 13835104.4 (86) International application number: PCT/JP2013/005359 (22) Date of filing: 10.09.2013 (87) International publication number: WO 2014/038216 (13.03.2014 Gazette 2014/11) (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • SATO, Eiji GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Yokohama-shi PL PT RO RS SE SI SK SM TR Kanagawa 227-8502 (JP) Designated Extension States: • YAMAZAKI, Michiko BA ME Yokohama-shi Kanagawa 227-8502 (JP) (30) Priority: 10.09.2012 JP 2012198840 • NAKAJIMA, Eiji 10.09.2012 JP 2012198841 Yokohama-shi 31.01.2013 JP 2013016947 Kanagawa 227-8502 (JP) 30.07.2013 JP 2013157306 • YU, Fujio 01.08.2013 JP 2013160301 Yokohama-shi 01.08.2013 JP 2013160300 Kanagawa 227-8502 (JP) 20.08.2013 JP 2013170404 • FUJITA, Toshio Yokohama-shi (83) Declaration under Rule 32(1) EPC (expert Kanagawa 227-8502 (JP) solution) • MIZUNASHI, Wataru Yokohama-shi (71) Applicant: Mitsubishi Rayon Co., Ltd. Kanagawa 227-8502 (JP) Tokyo 100-8253 (JP) (74) Representative: Hoffmann Eitle Patent- und Rechtsanwälte PartmbB Arabellastraße 30 81925 München (DE) (54) METHOD FOR PRODUCING METHACRYLIC ACID AND/OR ESTER THEREOF (57) To provide a method for directly and efficiently producing methacrylic acid in a single step from renew- able raw materials and/or biomass arising from the utili- zation of the renewable raw materials. -

Osher Lifelong Learning Institute
USDA-ARS National Plant Germplasm System Conservation of Fruit & Nut Genetic Resources Joseph Postman Plant Pathologist & Curator National Clonal Germplasm Repository Corvallis, Oregon May 2010 Mission: Collect – Preserve Evaluate – Enhance - Distribute World Diversity of Plant Genetic Resources for Improving the Quality and Production of Economic Crops Important to U.S. and World Agriculture Apple Accessions at Geneva Malus angustifolia ( 59 Accessions) Malus sikkimensis ( 14 Accessions) Malus baccata ( 67 Accessions) Malus sp. ( 41 Accessions) Malus bhutanica ( 117 Accessions) Malus spectabilis ( 9 Accessions) Malus brevipes ( 2 Accessions) Malus sylvestris ( 70 Accessions) Malus coronaria ( 98 Accessions) Malus toringo ( 122 Accessions) Malus domestica ( 1,389 Accessions) Malus transitoria ( 63 Accessions) Malus doumeri ( 2 Accessions) Malus trilobata ( 2 Accessions) Malus florentina ( 4 Accessions) Malus tschonoskii ( 3 Accessions) Malus floribunda ( 12 Accessions) Malus x adstringens ( 2 Accessions) Malus fusca ( 147 Accessions) Malus x arnoldiana ( 2 Accessions) Malus halliana ( 15 Accessions) Malus x asiatica ( 20 Accessions) Malus honanensis ( 4 Accessions) Malus x astracanica ( 1 Accessions) Malus hupehensis ( 185 Accessions) Malus x atrosanguinea ( 2 Accessions) Malus hybrid ( 337 Accessions) Malus x dawsoniana ( 2 Accessions) Malus ioensis ( 72 Accessions) Malus x hartwigii ( 5 Accessions) Malus kansuensis ( 45 Accessions) Malus x magdeburgensis ( 2 Accessions) Malus komarovii ( 1 Accessions) Malus x micromalus ( 25 Accessions) -

Native Trees of Georgia
1 NATIVE TREES OF GEORGIA By G. Norman Bishop Professor of Forestry George Foster Peabody School of Forestry University of Georgia Currently Named Daniel B. Warnell School of Forest Resources University of Georgia GEORGIA FORESTRY COMMISSION Eleventh Printing - 2001 Revised Edition 2 FOREWARD This manual has been prepared in an effort to give to those interested in the trees of Georgia a means by which they may gain a more intimate knowledge of the tree species. Of about 250 species native to the state, only 92 are described here. These were chosen for their commercial importance, distribution over the state or because of some unusual characteristic. Since the manual is intended primarily for the use of the layman, technical terms have been omitted wherever possible; however, the scientific names of the trees and the families to which they belong, have been included. It might be explained that the species are grouped by families, the name of each occurring at the top of the page over the name of the first member of that family. Also, there is included in the text, a subdivision entitled KEY CHARACTERISTICS, the purpose of which is to give the reader, all in one group, the most outstanding features whereby he may more easily recognize the tree. ACKNOWLEDGEMENTS The author wishes to express his appreciation to the Houghton Mifflin Company, publishers of Sargent’s Manual of the Trees of North America, for permission to use the cuts of all trees appearing in this manual; to B. R. Stogsdill for assistance in arranging the material; to W. -
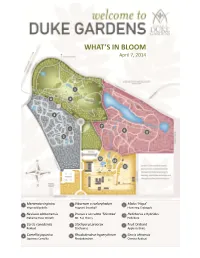
What's in Bloom
WHAT’S IN BLOOM April 7, 2014 5 4 6 2 7 1 9 8 3 12 10 11 1 Mertensia virginica 5 Viburnum x carlcephalum 9 Malus ‘Hopa’ Virginia Bluebells Fragrant Snowball Flowering Crabapple 2 Neviusia alabamensis 6 Prunus x serrulata ‘Shirotae’ 10 Helleborus x hybridus Alabama Snow Wreath Mt. Fuji Cherry Hellebore 3 Cercis canadensis 7 Stachyurus praecox 11 Fruit Orchard Redbud Stachyurus Apple cultivars 4 Camellia japonica 8 Rhododendron hyperythrum 12 Cercis chinensis Japanese Camellia Rhododendron Chinese Redbud WHAT’S IN BLOOM April 7, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Amelanchier arborea Common Serviceberry Sanguinaria canadensis Bloodroot Cornus florida Flowering Dogwood Stylophorum diphyllum Celandine Poppy Thalictrum thalictroides Rue Anemone Fothergilla major Fothergilla Trillium decipiens Chattahoochee River Trillium Hepatica nobilis Hepatica Trillium grandiflorum White Trillium Hexastylis virginica Wild Ginger Hexastylis minor Wild Ginger Trillium pusillum Dwarf Wakerobin Illicium floridanum Florida Anise Tree Trillium stamineum Blue Ridge Wakerobin Malus coronaria Sweet Crabapple Uvularia sessilifolia Sessileleaf Bellwort Mertensia virginica Virginia Bluebells Pachysandra procumbens Allegheny spurge Prunus americana American Plum DORIS DUKE CENTER GARDENS Camellia japonica Japanese Camellia Pulmonaria ‘Diana Clare’ Lungwort Cercis canadensis Redbud Prunus persica Flowering Peach Puschkinia scilloides Striped Squill Cercis chinensis Redbud Sanguinaria canadensis Bloodroot Clematis armandii Evergreen Clematis Spiraea prunifolia Bridalwreath -

Native Trees of Georgia.Pdf
NATIVE TREES OF GEORGIA By G. Norman Bishop Professor of Forestry George Foster Peabody School of Forestry University of Georgia Currently Named Daniel B. Warnell School of Forest Resources University of Georgia GEORGIA FORESTRY COMMISSION J. Frederick Allen Director Tenth Printing - 2000 Revised Edition 2 FOREWARD This manual has been prepared in an effort to give to those interested in the trees of Georgia a means by which they may gain a more intimate knowledge of the tree species. Of about 250 species native to the state, only 92 are described here. These were chosen for their commercial importance, distribution over the state or because of some unusual characteristic. Since the manual is intended primarily for the use of the layman, technical terms have been omitted wherever possible; however, the scientific names of the trees and the families to which they belong, have been included. It might be explained that the species are grouped by families, the name of each occurring at the top of the page over the name of the first member of that family. Also, there is included in the text, a subdivision entitled KEY CHARACTERISTICS, the purpose of which is to give the reader, all in one group, the most outstanding features whereby he may more easily recognize the tree. ACKNOWLEDGEMENTS The author wishes to express his appreciation to the Houghton Mifflin Company, publishers of Sargent’s Manual of the Trees of North America, for permission to use the cuts of all trees appearing in this manual; to B. R. Stogsdill for assistance in arranging the material; to W. -

Guidance Document for Tree Conservation, Landscape, and Buffer Requirements Article III Section 3.2
Guidance Document For Tree Conservation, Landscape, and Buffer Requirements Article III Section 3.2 Table of Contents Table of Contents Revision History ............................................................................................................................................ 1 Technical Standards ...................................................................................................................................... 2 1. Tree Measurements .......................................................................................................................... 2 2. Specimen Trees ................................................................................................................................. 4 3. Boundary Tree ................................................................................................................................... 4 4. Tree Density Calculation ................................................................................................................... 5 Tree Removal Requirements ...................................................................................................................... 11 Tree Care ..................................................................................................................................................... 12 1. Planting ........................................................................................................................................... 12 2. Mulching ........................................................................................................................................ -
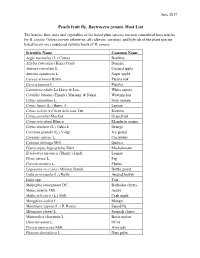
Peach Fruit Fly, Bactrocera Zonata, Host List the Berries, Fruit, Nuts and Vegetables of the Listed Plant Species Are Now Considered Host Articles for B
June 2017 Peach fruit fly, Bactrocera zonata, Host List The berries, fruit, nuts and vegetables of the listed plant species are now considered host articles for B. zonata. Unless proven otherwise, all cultivars, varieties, and hybrids of the plant species listed herein are considered suitable hosts of B. zonata. Scientific Name Common Name Aegle marmelos (L.) Correa Baeltree Afzelia xylocarpa (Kurz) Craib Doussie Annona reticulata L. Custard apple Annona squamosa L. Sugar apple Careya arborea Roxb. Patana oak Carica papaya L. Papaya Casimiroa edulis La Llave & Lex. White sapote Citrullus lanatus (Thunb.) Matsum. & Nakai Watermelon Citrus aurantium L. Sour orange Citrus limon (L.) Burm. f. Lemon Citrus nobilis x Citrus deliciosa Ten. Kinnow Citrus paradisi Macfad. Grapefruit Citrus reticulata Blanco Mandarin orange Citrus sinensis (L.) Osbeck Orange Coccinia grandis (L.) Voigt Ivy gourd Cucumis sativus L. Cucumber Cydonia oblonga Mill. Quince Elaeocarpus hygrophilus Kurz Ma-kok-nam Eriobotrya japonica (Thunb.) Lindl. Loquat Ficus carica L. Fig Grewia asiatica L. Phalsa Lagenaria siceraria (Molina) Standl. Bottle gourd Luffa acutangula (L.) Roxb. Angled loofah Luffa spp. Tori Malpighia emarginata DC. Barbados cherry Malus pumila Mill. Apple Malus sylvestris (L.) Mill. Crab apple Mangifera indica L. Mango Manilkara zapota (L.) P. Royen Sapodilla Mimusops elengi L. Spanish cherry Momordica charantia L. Bitter melon Olea europaea L. Olive Persea americana Mill. Avocado Phoenix dactylifera L. Date palm Prunus armeniaca L. Apricot Prunus avium (L.) L. Sweet cherry Prunus domestica L. Plum Prunus persica (L.) Batsch Peach Prunus sp. N/A Psidium cattleyanum Sabine Strawberry guava Psidium guajava L. Guava Punica granatum L. Pomegranate Putranjiva roxburghii Wall. -

Japanese Apple Rust Gymnosporangium Yamadae Belongs to the Pucciniales, an Order That Includes Rust Fungi That Cause Diseases on Plants
U.S. Department of Agriculture, Agricultural Research Service Systematic Mycology and Microbiology Laboratory - Invasive Fungi Fact Sheets Japanese apple rust Gymnosporangium yamadae belongs to the Pucciniales, an order that includes rust fungi that cause diseases on plants. Gymnosporangium yamadae has been limited in distribution to Asia (China, Japan and Korea) where it can be a serious pathogen of cultivated apples, especially if the host of the telial state, Juniperus spp., occurs in close proximity. This fungus was recently reported from the United States (DE, PA) in its aecial state on the ornamental tree, Malus toringo (Yun et al. 2009). Gymnosporangium yamadae Miyabe ex G. Yamada 1904 Aecia on Malus, foliicolous, then hypophyllous, also less commonly caulicolous, fructicolous; initially developing in a whitish leaf spot that becomes rose red with a distinct margin. Peridium cornute to tubular, 3-7(-8) mm high, retaining this shape at maturity but with lacerate sides that often form a reticulate pattern, apex typically closed to occasionally dehiscent, yellow-brown to brown; peridial cells long-narrow rhomboid to linear-rhomboid, 59-115 µm long, pale yellow, appearing verrucose with long papillae to tuberculate, outer walls smooth, inner and side walls sparsely echinulate. Aeciospores globoid, 16-26 × 18-27 µm, walls dark yellow, 1.0-2.5 µm thick, rarely up to 3.5 µm thick, sparsely echinulate, 4-7 pores scattered on surface. Telia on Juniperus, caulicolous, rarely foliicolous, produced on globoid swellings or small galls, telial horns cylindric-acuminate, 1-3 mm diam, 5-8 mm high or more, orange, gelatinouse. Teliospores two-celled, oblong, ellipsoid or obovoid, 15-28 x 32-56 µm, walls 0.8-2.0 µm thick, yellow or orange, with two pores near septum and one pore toward apex in upper cell, frequently with an obtuse, hyaline papilla at apex. -

'Fenghong Nichang' Flowering Crabapple
HORTSCIENCE 54(7):1260–1262. 2019. https://doi.org/10.21273/HORTSCI13897-19 Province of China. Because Malus seeds need cold stratification, we placed these seeds under moisture sand at –5 to 10 °C until ‘Fenghong Nichang’ Flowering radicles emerged. We then sowed them on raising beds in the field in Spring 2010 Crabapple at the National Crabapple Germplasm Ge- netic Center (Yangzhou, Jiangsu, China) Junjun Fan (Table 1). From 1,251 seedlings in 2012, College of Forestry, Nanjing Forestry University, Nanjing 210037, China; one double-flowered seedling was observed Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing and selected for further evaluation. In 2013 Forestry University, Nanjing 210037, China; and Department of Horticulture, and 2014, the plant consistently exhibited University of Georgia, Athens, GA 30602 15 to 25 petals per flower, and we named this individual plant ‘Fenghong Nichang’ Wangxiang Zhang (Fig. 1). From 2013 through 2015, we also College of Forestry, Nanjing Forestry University, Nanjing 210037, China; budded it into the popular rootstock, M. hupehensis, seedlings for 3 consecutive years. Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing In 2016 and 2017, 177 budded plants showed Forestry University, Nanjing 210037, China; and Yangzhou Crabapple the same flowering characteristics. From 2014 Horticulture Limited Company, Yangzhou 225200, China to 2015, we also conducted regional experi- ments in Shandong and Anhui Provinces Donglin Zhang (Table 1). ‘Fenghong Nichang’ showed the Department of Horticulture, University of Georgia, Athens, GA 30602 same flowering characteristics in these places. Malus halliana has a long cultiva- 1 Ting Zhou, Hao Jiang, Guibin Wang , and Fuliang Cao tion history in China. -

Tolerance of Apple and Peach Trees to Triclopyr
HORTSCIENCE 28(10):1021-1023. 1993. mine the extent of apple and peach tree toler- ance to triclopyr. Fruit trees have shown toler- ance to triclopyr, a postemergence herbicide Tolerance of Apple and Peach Trees to that controls important orchard broadleaf weeds, such as Virginia creeper (Young, 1989). Triclopyr Triclopyr at 0.1 to 1.12 kg acid equivalent (a.e.)/ha reduced ground cover by this weed in Jeffrey F. Derr peaches (Tworkoski and Young, 1990). Vir- Department of Plant Pathology, Physiology, and Weed Science, Virginia ginia creeper regrew, especially at the lower Polytechnic Institute and State University, Hampton Roads Agriculture triclopyr rates; however, control was accept- able with triclopyr applied at 1.12 kg·ha-1 for Experiment Station, Virginia Beach, VA 23455 two consecutive years. Triclopyr was equally Additional index words. Malus domestica, Prunus persica, crop injury, postemergence effective when applied in July, August, or herbicides, weed control September (Tworkoski and Young, 1989). Virginia creeper rooted near a tree trunk was Abstract. The tolerance of newly planted apple (Malus domestica Borkh.) and peach difficult to control since its foliage was pro- [Prunus persica (L.) Batsch] trees to the postemergence herbicide triclopyr was evaluated tected from the herbicide by the tree crown. infield trials. Apple and peach trees were not injured by triclopyr applied at rates ranging The ester formulation of triclopyr was more from 0.28 to 1.12 kg acid equivalent (a.e.)/ha as a directed spray to soil. No injury was effective than the amine formulation for Vir- observed following direct application of 10 ml of a triclopyr solution at 2 g a.e./liter to the ginia creeper control (Tworkoski et al., 1988). -

Landscaping Near Black Walnut Trees
Selecting juglone-tolerant plants Landscaping Near Black Walnut Trees Black walnut trees (Juglans nigra) can be very attractive in the home landscape when grown as shade trees, reaching a potential height of 100 feet. The walnuts they produce are a food source for squirrels, other wildlife and people as well. However, whether a black walnut tree already exists on your property or you are considering planting one, be aware that black walnuts produce juglone. This is a natural but toxic chemical they produce to reduce competition for resources from other plants. This natural self-defense mechanism can be harmful to nearby plants causing “walnut wilt.” Having a walnut tree in your landscape, however, certainly does not mean the landscape will be barren. Not all plants are sensitive to juglone. Many trees, vines, shrubs, ground covers, annuals and perennials will grow and even thrive in close proximity to a walnut tree. Production and Effect of Juglone Toxicity Juglone, which occurs in all parts of the black walnut tree, can affect other plants by several means: Stems Through root contact Leaves Through leakage or decay in the soil Through falling and decaying leaves When rain leaches and drips juglone from leaves Nuts and hulls and branches onto plants below. Juglone is most concentrated in the buds, nut hulls and All parts of the black walnut tree produce roots and, to a lesser degree, in leaves and stems. Plants toxic juglone to varying degrees. located beneath the canopy of walnut trees are most at risk. In general, the toxic zone around a mature walnut tree is within 50 to 60 feet of the trunk, but can extend to 80 feet. -

Those Amazing Magnolia Fruits Richard B
(sr or 72 MAGIVOIIA Those Amazing Magnolia Fruits Richard B. Fig far Unlike people who are interested in growing nut trees like Juglans or fruit trees such as Malus, those who cultivate magnolias are mainly interested in the flowers, not the fruits. Though some fruits of mag- nolia species are quite ornamental, I find the fruits to be most useful for studying the differences (or similarities) between species or groups of species of Magnolias. Taxonomists have long had a similar interest in observing fruits of Magnoliaceae and they have often used those differences or perceived differences in fruit characters to justify their systems for classification of Magnoliaceae. When James E. Dandy codified his system of Magnoliaceae in 1927, he based much of his classification on fruit characters. This basis remained virtually unchanged for the rest of his life, for example (adapted from Dandy r964); A. Fruiting carpels dehiscent, not fleshy, B. Carpels free, in fruit dehiscent along the dorsal suture, C. Ovules 4 or more in each carpel . .. .. .. .. .. .. .. .. Manglietia C. Ovules a in each carpel (rarely 3-4 in the lower carpels) .. Magnolia B. Carpels concrescent at least at the base, in fruit circumscissile and woody, the upper portions falling away either singly or in irregular masses, the lower portions persistent with suspended seeds; stipules adnate to the petiole . Talauma A. Fruiting carpels indehiscent, concrescent to form a fleshy syncarp; etc Aromadendron As new species were discovered, taxonomists often followed Dandy's guidelines regarding fruit characters, which resulted in the creation of even more Magnoliaceae genera based on relatively minor varia- tions in the fruits.