Aquatic Insect Ecophysiological Traits Reveal Phylogenetically Based Differences in Dissolved Cadmium Susceptibility
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The 2014 Golden Gate National Parks Bioblitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 ON THIS PAGE Photograph of BioBlitz participants conducting data entry into iNaturalist. Photograph courtesy of the National Park Service. ON THE COVER Photograph of BioBlitz participants collecting aquatic species data in the Presidio of San Francisco. Photograph courtesy of National Park Service. The 2014 Golden Gate National Parks BioBlitz - Data Management and the Event Species List Achieving a Quality Dataset from a Large Scale Event Natural Resource Report NPS/GOGA/NRR—2016/1147 Elizabeth Edson1, Michelle O’Herron1, Alison Forrestel2, Daniel George3 1Golden Gate Parks Conservancy Building 201 Fort Mason San Francisco, CA 94129 2National Park Service. Golden Gate National Recreation Area Fort Cronkhite, Bldg. 1061 Sausalito, CA 94965 3National Park Service. San Francisco Bay Area Network Inventory & Monitoring Program Manager Fort Cronkhite, Bldg. 1063 Sausalito, CA 94965 March 2016 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate comprehensive information and analysis about natural resources and related topics concerning lands managed by the National Park Service. -

Monte L. Bean Life Science Museum Brigham Young University Provo, Utah 84602 PBRIA a Newsletter for Plecopterologists
No. 10 1990/1991 Monte L. Bean Life Science Museum Brigham Young University Provo, Utah 84602 PBRIA A Newsletter for Plecopterologists EDITORS: Richard W, Baumann Monte L. Bean Life Science Museum Brigham Young University Provo, Utah 84602 Peter Zwick Limnologische Flußstation Max-Planck-Institut für Limnologie, Postfach 260, D-6407, Schlitz, West Germany EDITORIAL ASSISTANT: Bonnie Snow REPORT 3rd N orth A merican Stonefly S ymposium Boris Kondratieff hosted an enthusiastic group of plecopterologists in Fort Collins, Colorado during May 17-19, 1991. More than 30 papers and posters were presented and much fruitful discussion occurred. An enjoyable field trip to the Colorado Rockies took place on Sunday, May 19th, and the weather was excellent. Boris was such a good host that it was difficult to leave, but many participants traveled to Santa Fe, New Mexico to attend the annual meetings of the North American Benthological Society. Bill Stark gave us a way to remember this meeting by producing a T-shirt with a unique “Spirit Fly” design. ANNOUNCEMENT 11th International Stonefly Symposium Stan Szczytko has planned and organized an excellent symposium that will be held at the Tree Haven Biological Station, University of Wisconsin in Tomahawk, Wisconsin, USA. The registration cost of $300 includes lodging, meals, field trip and a T- Shirt. This is a real bargain so hopefully many colleagues and friends will come and participate in the symposium August 17-20, 1992. Stan has promised good weather and good friends even though he will not guarantee that stonefly adults will be collected during the field trip. Printed August 1992 1 OBITUARIES RODNEY L. -

Download .PDF(1340
Stark, Bill P. and Stephen Green. 2011. Eggs of western Nearctic Acroneuriinae (Plecoptera: Perlidae). Illiesia, 7(17):157-166. Available online: http://www2.pms-lj.si/illiesia/Illiesia07-17.pdf EGGS OF WESTERN NEARCTIC ACRONEURIINAE (PLECOPTERA: PERLIDAE) Bill P. Stark1 and Stephen Green2 1,2 Box 4045, Department of Biology, Mississippi College, Clinton, Mississippi, U.S.A. 39058 1 E-mail: [email protected] 2 E-mail: [email protected] ABSTRACT Eggs for western Nearctic acroneuriine species of Calineuria Ricker, Doroneuria Needham & Claassen and Hesperoperla Banks are examined and redescribed based on scanning electron microscopy images taken from specimens collected from a substantial portion of each species range. Within genera, species differences in egg morphology are small and not always useful for species recognition, however eggs from one population of Calineuria are significantly different from those found in other populations and this population is given informal recognition as a possible new species. Keywords: Plecoptera, Calineuria, Doroneuria, Hesperoperla, Egg morphology, Western Nearctic INTRODUCTION occur in the region (Baumann & Olson 1984; Scanning electron microscopy (SEM) is often used Kondratieff & Baumann 2002; Stark 1989; Stark & to elucidate chorionic features for stoneflies (e.g. Gaufin 1976; Stark & Kondratieff 2004; Zuellig et al. Baumann 1973; Grubbs 2005; Isobe 1988; Kondratieff 2006). SEM images for eggs of the primary western 2004; Kondratieff & Kirchner 1996; Nelson 2000; acroneuriine genera, Calineuria Ricker, Doroneuria Sivec & Stark 2002; 2008; Stark & Nelson 1994; Stark Needham & Claassen and Hesperoperla Banks include & Szczytko 1982; 1988; Szczytko & Stewart 1979) and single images for each of these genera in Stark & Nearctic Perlidae were among the earliest stoneflies Gaufin (1976), three images of Hesperoperla hoguei to be studied with this technique (Stark & Gaufin Baumann & Stark (1980) and three images of H. -

List of Animal Species with Ranks October 2017
Washington Natural Heritage Program List of Animal Species with Ranks October 2017 The following list of animals known from Washington is complete for resident and transient vertebrates and several groups of invertebrates, including odonates, branchipods, tiger beetles, butterflies, gastropods, freshwater bivalves and bumble bees. Some species from other groups are included, especially where there are conservation concerns. Among these are the Palouse giant earthworm, a few moths and some of our mayflies and grasshoppers. Currently 857 vertebrate and 1,100 invertebrate taxa are included. Conservation status, in the form of range-wide, national and state ranks are assigned to each taxon. Information on species range and distribution, number of individuals, population trends and threats is collected into a ranking form, analyzed, and used to assign ranks. Ranks are updated periodically, as new information is collected. We welcome new information for any species on our list. Common Name Scientific Name Class Global Rank State Rank State Status Federal Status Northwestern Salamander Ambystoma gracile Amphibia G5 S5 Long-toed Salamander Ambystoma macrodactylum Amphibia G5 S5 Tiger Salamander Ambystoma tigrinum Amphibia G5 S3 Ensatina Ensatina eschscholtzii Amphibia G5 S5 Dunn's Salamander Plethodon dunni Amphibia G4 S3 C Larch Mountain Salamander Plethodon larselli Amphibia G3 S3 S Van Dyke's Salamander Plethodon vandykei Amphibia G3 S3 C Western Red-backed Salamander Plethodon vehiculum Amphibia G5 S5 Rough-skinned Newt Taricha granulosa -

New Records of Stoneflies (Plecoptera) with an Annotated Checklist of the Species for Pennsylvania
The Great Lakes Entomologist Volume 29 Number 3 - Fall 1996 Number 3 - Fall 1996 Article 2 October 1996 New Records of Stoneflies (Plecoptera) With an Annotated Checklist of the Species for Pennsylvania E. C. Masteller Behrend College Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Masteller, E. C. 1996. "New Records of Stoneflies (Plecoptera) With an Annotated Checklist of the Species for Pennsylvania," The Great Lakes Entomologist, vol 29 (3) Available at: https://scholar.valpo.edu/tgle/vol29/iss3/2 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Masteller: New Records of Stoneflies (Plecoptera) With an Annotated Checklis 1996 THE GREAT LAKES ENTOMOlOGIST 107 NEW RECORDS OF STONEFLIES IPLECOPTERA} WITH AN ANNOTATED CHECKLIST OF THE SPECIES FOR PENNSYLVANIA E.C. Masteller1 ABSTRACT Original collections now record 134 species in nine families and 42 gen era. Seventeen new state records include, Allocapnia wrayi, Alloperla cau data, Leuctra maria, Soyedina carolinensis, Tallaperla elisa, Perlesta decipi· ens, P. placida, Neoperla catharae, N. occipitalis, N. stewarti, Cult us decisus decisus, Isoperla francesca, 1. frisoni, 1. lata,1. nana, 1. slossonae, Malirekus hastatus. Five species are removed from the list ofspecies for Pennsylvania. Surdick and Kim (1976) originally recorded 90 species of stoneflies in nine families and 32 genera from Pennsylvania. Since that time, Stark et al. -
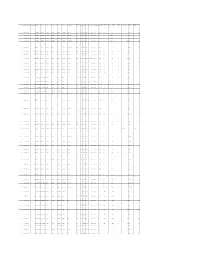
Appendix D: Summary of Accepted ECOTOX Papers
Effect Dur Dur Unit Dur Dur Unit Conc Conc Units Conc Value1 Purity CAS Number Chemical Name Species Number Phylum Class Order Family Genus Species Common Name Group Effect Meas Endpt1 Endpt2 Habitat Plant/Animal Media Orig Orig Preferred Preferred Type Conc Value1 Orig Orig Adjusted 1 298044 Disulfoton 4510 Chordata Mammalia Rodentia Muridae Rattus norvegicus Norway rat BEH AVO STIM NOAEL terrestrial Animal NONE 30 d 30 d F 2 mg/kg/d 1.96 2 298044 Disulfoton 351 Chordata Actinopterygii Perciformes Anabantidae Anabas testudineus Climbing perch BCM BCM LIPD LOAEL aquatic Animal FW 1 h 4.17E-02 d A 4 mg/L 4 3 298044 Disulfoton 351 Chordata Actinopterygii Perciformes Anabantidae Anabas testudineus Climbing perch BCM BCM PRCO LOAEL aquatic Animal FW 1 h 4.17E-02 d A 4 mg/L 4 4 298044 Disulfoton 351 Chordata Actinopterygii Perciformes Anabantidae Anabas testudineus Climbing perch BCM BCM LIPD LOAEL aquatic Animal FW 1 h 4.17E-02 d A 10.5 mg/L 10.5 5 298044 Disulfoton 4913 Chordata Mammalia Rodentia Muridae Mus musculus House mouse BCM BCM HXBT LOAEL terrestrial Animal NONE 3 d 3 d F 35.1 uM/kg 35.1 6 298044 Disulfoton 4510 Chordata Mammalia Rodentia Muridae Rattus norvegicus Norway rat BCM BCM GBCM LOAEL terrestrial Animal NONE 24 h 1 d F 0.26 mg/kg 0.234 7 298044 Disulfoton 4510 Chordata Mammalia Rodentia Muridae Rattus norvegicus Norway rat BCM BCM GBCM IC50 terrestrial Animal NONE 1 wk 7 d F 0.6 ppm 0.6 8 298044 Disulfoton 4510 Chordata Mammalia Rodentia Muridae Rattus norvegicus Norway rat BCM BCM GBCM NOAEL LOAEL terrestrial Animal NONE -

Comprehensive Conservation Plan Benton Lake National Wildlife
Glossary accessible—Pertaining to physical access to areas breeding habitat—Environment used by migratory and activities for people of different abilities, es- birds or other animals during the breeding sea- pecially those with physical impairments. son. A.D.—Anno Domini, “in the year of the Lord.” canopy—Layer of foliage, generally the uppermost adaptive resource management (ARM)—The rigorous layer, in a vegetative stand; mid-level or under- application of management, research, and moni- story vegetation in multilayered stands. Canopy toring to gain information and experience neces- closure (also canopy cover) is an estimate of the sary to assess and change management activities. amount of overhead vegetative cover. It is a process that uses feedback from research, CCP—See comprehensive conservation plan. monitoring, and evaluation of management ac- CFR—See Code of Federal Regulations. tions to support or change objectives and strate- CO2—Carbon dioxide. gies at all planning levels. It is also a process in Code of Federal Regulations (CFR)—Codification of which the Service carries out policy decisions the general and permanent rules published in the within a framework of scientifically driven ex- Federal Register by the Executive departments periments to test predictions and assumptions and agencies of the Federal Government. Each inherent in management plans. Analysis of re- volume of the CFR is updated once each calendar sults helps managers decide whether current year. management should continue as is or whether it compact—Montana House bill 717–Bill to Ratify should be modified to achieve desired conditions. Water Rights Compact. alternative—Reasonable way to solve an identi- compatibility determination—See compatible use. -

Empirically Derived Indices of Biotic Integrity for Forested Wetlands, Coastal Salt Marshes and Wadable Freshwater Streams in Massachusetts
Empirically Derived Indices of Biotic Integrity for Forested Wetlands, Coastal Salt Marshes and Wadable Freshwater Streams in Massachusetts September 15, 2013 This report is the result of several years of field data collection, analyses and IBI development, and consideration of the opportunities for wetland program and policy development in relation to IBIs and CAPS Index of Ecological Integrity (IEI). Contributors include: University of Massachusetts Amherst Kevin McGarigal, Ethan Plunkett, Joanna Grand, Brad Compton, Theresa Portante, Kasey Rolih, and Scott Jackson Massachusetts Office of Coastal Zone Management Jan Smith, Marc Carullo, and Adrienne Pappal Massachusetts Department of Environmental Protection Lisa Rhodes, Lealdon Langley, and Michael Stroman Empirically Derived Indices of Biotic Integrity for Forested Wetlands, Coastal Salt Marshes and Wadable Freshwater Streams in Massachusetts Abstract The purpose of this study was to develop a fully empirically-based method for developing Indices of Biotic Integrity (IBIs) that does not rely on expert opinion or the arbitrary designation of reference sites and pilot its application in forested wetlands, coastal salt marshes and wadable freshwater streams in Massachusetts. The method we developed involves: 1) using a suite of regression models to estimate the abundance of each taxon across a gradient of stressor levels, 2) using statistical calibration based on the fitted regression models and maximum likelihood methods to predict the value of the stressor metric based on the abundance of the taxon at each site, 3) selecting taxa in a forward stepwise procedure that conditionally improves the concordance between the observed stressor value and the predicted value the most and a stopping rule for selecting taxa based on a conditional alpha derived from comparison to pseudotaxa data, and 4) comparing the coefficient of concordance for the final IBI to the expected distribution derived from randomly permuted data. -

Plecoptera: Perlidae), with an Annotated Checklist of the Subfamily in the Realm
Opusc. Zool. Budapest, 2016, 47(2): 173–196 On the identity of some Oriental Acroneuriinae taxa (Plecoptera: Perlidae), with an annotated checklist of the subfamily in the realm D. MURÁNYI1 & W.H. LI2 1Dávid Murányi, Department of Civil and Environmental Engineering, Ehime University, Bunkyo-cho 3, Matsuyama, 790-8577 Japan, and Department of Zoology, Hungarian Natural History Museum, H-1088 Budapest, Baross u. 13, Hungary. E-mail: [email protected], [email protected] 2Weihai Li, Department of Plant Protection, Henan Institute of Science and Technology, Xinxiang, Henan, 453003 China. E-mail: [email protected] Abstract. The monotypic Taiwanese genus Mesoperla Klapálek, 1913 is redescribed on the basis of a male syntype specimen, and its affinities are re-evaluated. The single female type specimen of further two Oriental monotypic genera, Kalidasia Klapálek, 1914 and Nirvania Klapálek, 1914, are confirmed to be lost or destroyed respectively; both genera are considered as nomina dubia. The Sichuan endemic Acroneuria grahami Wu & Claassen, 1934 is redescribed on the basis of male holotype. Distinctive characters of the genus Brahmana Klapálek, 1914 consisting of five, inadequately known Oriental species are discussed. Flavoperla needhami (Klapálek, 1916) and Sinacroneuria sinica (Yang & Yang, 1998) comb. novae are suggested for an Indian species originally described in Gibosia Okamoto, 1912 and a Chinese species originally described in Acroneuria Pictet, 1841. At present, 62 species of Acroneuriinae, classified in 10 valid genera are reported from the Oriental Realm but 29 species are inadequately known. A key is presented to distinguish males of the Asian Acroneuriinae genera. Asian distribution of each genera are detailed and depicted on a map. -

The Life History, Nymphal Growth Rates, and Feeding Habits of Siphlonisca Aerodromia Needham (Epherneroptera: Siphlonuridae) in ~Aine'
The life history, nymphal growth rates, and feeding habits of Siphlonisca aerodromia Needham (Epherneroptera: Siphlonuridae) in ~aine' K. ELIZABETHGIBBS AND TERRYM. MINGO Department of Entomology, University of Maine, Orono, ME, U. S. A. 04469 Received March 25. 1985 GIBBS,K. E., and T. M. MINGO.1986. The life history, nymphal growth rates, and feeding habits of Siphlonisca aerodromia Needham (Epherneroptera: Siphlonuridae) in Maine. Can. J. Zool. 64: 427-430. Siphlonisca aerodromia Needham has a univoltine life history in Maine. Adults emerge in late May or early June. Each female contains about 394 large (0.46 mm long) eggs covered with coiled fibers that anchor the eggs to the substrate. Eggs are deposited in the main channel of the stream and small nymphs appear in January. Nymphal growth rate (GHW)was expressed as a percent per day increase in head width. Initially nymphs feed on detritus and grow slowly (GHW= 0.28-0.79) at water temperatures near 0°C. Following snow melt, the nymphs move into the adjacent Carex floodplain. Here, water temperature increases, animal material, in the form of mayfly nymphs, becomes increasingly common in the diet, and growth rate increases (GHW = 2.13-2.89). The sex ratio of nymphs collected in May and June was 1: 1.8 (ma1e:female). GIBBS,K. E., et T. M. MINGO. 1986. The life history, nymphal growth rates, and feeding habits of Siphlonisca aerodromia Needham (Epherneroptera: Siphlonuridae) in Maine. Can. J. Zool. 64: 427-430. Dans le Maine, le cycle de Siphlonisca aerodromia Needham est univoltin. L'emergence des adultes se produit a la fin de mai ou au debut de juin. -

The Effects of Electricity on Some Aquatic Invertebrates
The effects of electricity on some aquatic invertebrates Item Type text; Thesis-Reproduction (electronic) Authors Mesick, Carl Frederick Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 26/09/2021 21:59:29 Link to Item http://hdl.handle.net/10150/558075 THE EFFECTS OF ELECTRICITY ON SOME AQUATIC INVERTEBRATES by Carl Frederick Mesick A Thesis Submitted to the Faculty of the SCHOOL OF RENEWABLE NATURAL RESOURCES In Partial Fulfillment of Requirements For the Degree of MASTER OF SCIENCE WITH A MAJOR IN FISHERIES SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 1 9 7 9 STATEMENT BY AUTHOR This thesis has been submitted in partial fulfill ment of requirements for an advanced degree at The University of Arizona and is deposited in the University Library to be made available to borrowers under rules of the Libraryo Brief quotations from this thesis are allowable without special permission, provided that accurate acknowl edgment of source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the head of the major department or the Dean of the Graduate College when in his judgment the proposed use of the material is in the inter ests of scholarship. In all other instances, however, permission must be obtained from the author, SIGNED i ' ^ V[ APPROVAL BY THESIS DIRECTOR This thesis has been approved on the date shown below: JERRY C . -

TB142: Mayflies of Maine: an Annotated Faunal List
The University of Maine DigitalCommons@UMaine Technical Bulletins Maine Agricultural and Forest Experiment Station 4-1-1991 TB142: Mayflies of aine:M An Annotated Faunal List Steven K. Burian K. Elizabeth Gibbs Follow this and additional works at: https://digitalcommons.library.umaine.edu/aes_techbulletin Part of the Entomology Commons Recommended Citation Burian, S.K., and K.E. Gibbs. 1991. Mayflies of Maine: An annotated faunal list. Maine Agricultural Experiment Station Technical Bulletin 142. This Article is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Technical Bulletins by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. ISSN 0734-9556 Mayflies of Maine: An Annotated Faunal List Steven K. Burian and K. Elizabeth Gibbs Technical Bulletin 142 April 1991 MAINE AGRICULTURAL EXPERIMENT STATION Mayflies of Maine: An Annotated Faunal List Steven K. Burian Assistant Professor Department of Biology, Southern Connecticut State University New Haven, CT 06515 and K. Elizabeth Gibbs Associate Professor Department of Entomology University of Maine Orono, Maine 04469 ACKNOWLEDGEMENTS Financial support for this project was provided by the State of Maine Departments of Environmental Protection, and Inland Fisheries and Wildlife; a University of Maine New England, Atlantic Provinces, and Quebec Fellow ship to S. K. Burian; and the Maine Agricultural Experiment Station. Dr. William L. Peters and Jan Peters, Florida A & M University, pro vided support and advice throughout the project and we especially appreci ated the opportunity for S.K. Burian to work in their laboratory and stay in their home in Tallahassee, Florida.