United States Patent Office Patented July 31, 1956
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Heat of Combustion of Beryllium in Fluorine*
JOURNAL OF RESEARCH of the National Bureau of Standards -A. Physics and Chemistry Vol. 73A, No.3, May- June 1969 The Heat of Combustion of Beryllium in Fluorine* K. L. Churney and G. T. Armstrong Institute for Materials Research, National Bureau of Standards, Washington, D.C. 20234 (February 11, 1969) An expe rimental dete rmination of the e ne rgies of combustion in Auorine of polyte traAuoroethylene film and Q.o wder and of mixtures of beryllium with polytetraAuoroethyle ne gi ves for reacti on ( 1)f).H ~.or= - 1022.22 kJ 111 0 1- 1 (- 244.32 kcal mol - I) wit h a n ove ra ll precision of 0.96 kJ 111 0 1- 1 (0. 23 kcal 111 0 1- 1 ) at the 95 pe rce nt confid ence limit s. The tota l un cert a int y is estimated not to exceed ±3.2 kJ mol- I (±0.8 kcal mol - I). The measureme nts on polytetraflu oroeth yle ne giv e for reaction (2a) and reacti on (2 b) f).H ~. o c =- 10 369. 7 and - 10392.4 Jg- I, respective ly. Overall precisions e xpressed at the 95 pe rcent confide nce Ijmits are 3.3 and 6.0 Jg- I, respective ly. Be(c)+ F,(g) = BeF2(a morphous) (1) C,F.(polym e r powd er) + 2F2(g) = 2CF.(g) (2a) C2F.(polyme r film ) + 2F2 (g) = 2CF.(g) (2b) Be2C and Be metal were observed in a small carbonaceous residue from the co mbustion of the beryll iul11 -polytetraAuoroethylene mixtures. -
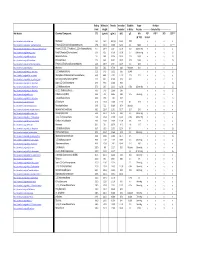
Detectable Compounds by MIP.Pdf
Boiling Molecular Density Ionization Solubility Vapor Analytes Point Weight Potential in Water Pressure ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐Detected By‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Wiki Website Chemical Compound: (oC) (g/mol) (g/mL) (eV) g/L kPa PID* FID** XSD ECD*** @ ~25C 10.6 eV https://en.wikipedia.org/wiki/Methane . Methane ‐161 16.0 .657 g/L 12.61 0.02 n y n n https://en.wikipedia.org/wiki/Dichlorodifluoromethane . Freon 12 (Dichlorodifluoromethane) ‐29.8 120.9 1.486 12.31 0.3 568.0 n y y LS**** https://en.wikipedia.org/wiki/1,1,2‐Trichloro‐1,2,2‐trifluoroethane . Freon 113 (1,1,2‐Trichloro‐1,2,2‐trifluoroethane) 47.7 187.4 1.564 11.78 0.17 285mm Hg n y y y https://en.wikipedia.org/wiki/Vinyl_chloride . Vinyl Chloride (Chloroethene) ‐13.0 62.5 0.910 10.00 2.7 2580mm Hg y y y LS https://en.wikipedia.org/wiki/Bromomethane . Bromomethane 3.6 94.9 3.974 10.53 17.5 190.0 n y y LS https://en.wikipedia.org/wiki/Chloroethane .. Chloroethane 12.3 64.5 0.901 10.97 5.74 134.6 n y y LS https://en.wikipedia.org/wiki/Trichlorofluoromethane . Freon 11 (Trichlorofluoromethane) 23.8 137.4 1.494 11.77 1.1 89.0 n y y y https://en.wikipedia.org/wiki/Acetone . Acetone 56.5 58.1 0.790 9.69 Miscible 30.6 y y n n https://en.wikipedia.org/wiki/1,1‐Dichloroethene . 1,1‐Dichloroethene 32.0 97.0 1.213 9.65 0.04% y y y LS https://en.wikipedia.org/wiki/Dichloromethane . -

1,1,1,2-Tetrafluoroethane
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organisation, or the World Health Organization. Concise International Chemical Assessment Document 11 1,1,1,2-Tetrafluoroethane First draft prepared by Mrs P. Barker and Mr R. Cary, Health and Safety Executive, Liverpool, United Kingdom, and Dr S. Dobson, Institute of Terrestrial Ecology, Huntingdon, United Kingdom Please not that the layout and pagination of this pdf file are not identical to the printed CICAD Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organisation, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 1998 The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organisation (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. The Inter-Organization -

SAFETY DATA SHEET Difluoromethane (R32) SECTION 1
SAFETY DATA SHEET Difluoromethane (R32) Issue Date: 16.01.2013 Version: 1.1 SDS No.: 000010021734 Last revised date: 26.11.2018 1/14 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Product name: Difluoromethane (R32) Other Name: HFC-32 Additional identification Chemical name: Difluoromethane Chemical formula: CH2F2 INDEX No. - CAS-No. 75-10-5 EC No. 200-839-4 REACH Registration No. 01-2119471312-47 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Industrial and professional. Perform risk assessment prior to use. Refrigerant. Use as an Intermediate (transported, on-site isolated). Use for electronic component manufacture. Using gas alone or in mixtures for the calibration of analysis equipment. Formulation of mixtures with gas in pressure receptacles. Uses advised against Consumer use. 1.3 Details of the supplier of the safety data sheet Supplier Linde Gas GmbH Telephone: +43 50 4273 Carl-von-Linde-Platz 1 A-4651 Stadl-Paura E-mail: [email protected] 1.4 Emergency telephone number: Emergency number Linde: + 43 50 4273 (during business hours), Poisoning Information Center: +43 1 406 43 43 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Regulation (EC) No 1272/2008 as amended. Physical Hazards Flammable gas Category 1 H220: Extremely flammable gas. Gases under pressure Liquefied gas H280: Contains gas under pressure; may explode if heated. SDS_AT - 000010021734 SAFETY DATA SHEET Difluoromethane (R32) Issue Date: 16.01.2013 Version: 1.1 SDS No.: 000010021734 Last revised date: 26.11.2018 2/14 2.2 Label Elements Signal Words: Danger Hazard Statement(s): H220: Extremely flammable gas. -

Dichlorodifluoromethane Dcf
DICHLORODIFLUOROMETHANE DCF CAUTIONARY RESPONSE INFORMATION 4. FIRE HAZARDS 7. SHIPPING INFORMATION 4.1 Flash Point: 7.1 Grades of Purity: 99.5% (vol.) Common Synonyms Gas Colorless Faint odor Not flammable 7.2 Storage Temperature: Ambient Arcton 6 4.2 Flammable Limits in Air: Not flammable Eskimon 12 7.3 Inert Atmosphere: No requirement 4.3 Fire Extinguishing Agents: Not F-12 Visible vapor cloud is produced. 7.4 Venting: Safety relief flammable Freon 12 7.5 IMO Pollution Category: Currently not available Frigen 12 4.4 Fire Extinguishing Agents Not to Be Genetron 12 Used: Not flammable 7.6 Ship Type: Currently not available Halon 122 4.5 Special Hazards of Combustion 7.7 Barge Hull Type: 3 Isotron 12 Products: Although nonflammable, Ucon 12 dissociation products generated in a fire may be irritating or toxic. 8. HAZARD CLASSIFICATIONS Notify local health and pollution control agencies. 4.6 Behavior in Fire: Helps extinguish fire. 8.1 49 CFR Category: Nonflammable gas Avoid inhalation. 4.7 Auto Ignition Temperature: Not 8.2 49 CFR Class: 2.2 flammable 8.3 49 CFR Package Group: Not pertinent. Not flammable. 4.8 Electrical Hazards: Not pertinent Fire Cool exposed containers with water. 8.4 Marine Pollutant: No 4.9 Burning Rate: Not flammable 8.5 NFPA Hazard Classification: Not listed 4.10 Adiabatic Flame Temperature: Currently 8.6 EPA Reportable Quantity: 5000 pounds CALL FOR MEDICAL AID. not available Exposure 8.7 EPA Pollution Category: D 4.11 Stoichometric Air to Fuel Ratio: Not VAPOR 8.8 RCRA Waste Number: U075 Not irritating to eyes, nose or throat. -

Exposure Investigation Protocol: the Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH
Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan Exposure Investigation Protocol: The Identification of Air Contaminants Around the Continental Aluminum Plant in New Hudson, Michigan Conducted by ATSDR and MDCH MDCH/ATSDR - 2004 Exposure Investigation Protocol - Continental Aluminum New Hudson, Lyon Township, Oakland County, Michigan TABLE OF CONTENTS OBJECTIVE/PURPOSE..................................................................................................... 3 RATIONALE...................................................................................................................... 4 BACKGROUND ................................................................................................................ 5 AGENCY ROLES .............................................................................................................. 6 ESTABLISHING CRITERIA ............................................................................................ 7 “Odor Events”................................................................................................................. 7 Comparison Values......................................................................................................... 7 METHODS ....................................................................................................................... 12 Instantaneous (“Grab”) Air Sampling........................................................................... 12 Continuous Air Monitoring.......................................................................................... -

SAFETY DATA SHEET Halocarbon R-503
SAFETY DATA SHEET Halocarbon R-503 Section 1. Identification GHS product identifier : Halocarbon R-503 Other means of : Not available. identification Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. SDS # : 007306 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture HAZARDOUS TO THE OZONE LAYER - Category 1 GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Harms public health and the environment by destroying ozone in the upper atmosphere. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Always keep container in upright position. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Refer to manufacturer or supplier for information on recovery or recycling. Hazards not otherwise : Liquid can cause burns similar to frostbite. -

Cylinder Valve Selection Quick Reference for Valve Abbreviations
SHERWOOD VALVE COMPRESSED GAS PRODUCTS Appendix Cylinder Valve Selection Quick Reference for Valve Abbreviations Use the Sherwood Cylinder Valve Series Abbreviation Chart on this page with the Sherwood Cylinder Valve Selection Charts found on pages 73–80. The Sherwood Cylinder Valve Selection Chart are for reference only and list: • The most commonly used gases • The Compressed Gas Association primary outlet to be used with each gas • The Sherwood valves designated for use with this gas • The Pressure Relief Device styles that are authorized by the DOT for use with these gases PLEASE NOTE: The Sherwood Cylinder Valve Selection Charts are partial lists extracted from the CGA V-1 and S-1.1 pamphlets. They can change without notice as the CGA V-1 and S-1.1 pamphlets are amended. Sherwood will issue periodic changes to the catalog. If there is any discrepancy or question between these lists and the CGA V-1 and S-1.1 pamphlets, the CGA V-1 and S-1.1 pamphlets take precedence. Sherwood Cylinder Valve Series Abbreviation Chart Abbreviation Sherwood Valve Series AVB Small Cylinder Acetylene Wrench-Operated Valves AVBHW Small Cylinder Acetylene Handwheel-Operated Valves AVMC Small Cylinder Acetylene Wrench-Operated Valves AVMCHW Small Cylinder Acetylene Handwheel-Operated Valves AVWB Small Cylinder Acetylene Wrench-Operated Valves — WB Style BV Hi/Lo Valves with Built-in Regulator DF* Alternative Energy Valves GRPV Residual Pressure Valves GV Large Cylinder Acetylene Valves GVT** Vertical Outlet Acetylene Valves KVAB Post Medical Valves KVMB Post Medical Valves NGV Industrial and Chrome-Plated Valves YVB† Vertical Outlet Oxygen Valves 1 * DF Valves can be used with all gases; however, the outlet will always be ⁄4"–18 NPT female. -

Refrigerant Safety Refrigerant History
Refrigerant Safety The risks associated with the use of refrigerants in refrigeration and airconditioning equipment can include toxicity, flammability, asphyxiation, and physical hazards. Although refrigerants can pose one or more of these risks, system design, engineering controls, and other techniques mitigate this risk for the use of refrigerant in various types of equipment. Refrigerant History Nearly all of the historically used refrigerants were flammable, toxic, or both. Some were also highly reactive, resulting in accidents (e. g., leak, explosion) due to equipment failure, poor maintenance, or human error. The task of finding a nonflammable refrigerant with good stability was given to Thomas Midgley in 1926. With his associates Albert Leon Henne and Robert Reed McNary, Dr. Midgley observed that the refrigerants then in use comprised relatively few chemical elements, many of which were clustered in an intersecting row and column of the periodic table of elements. The element at the intersection was fluorine, known to be toxic by itself. Midgley and his collaborators felt, however, that compounds containing fluorine could be both nontoxic and nonflammable. The attention of Midgley and his associates was drawn to organic fluorides by an error in the literature that showed the boiling point for tetrafluoromethane (carbon tetrafluoride) to be high compared to those for other fluorinated compounds. The correct boiling temperature later was found to be much lower. Nevertheless, the incorrect value was in the range sought and led to evaluation of organic fluorides as candidates. The shorthand convention, later introduced to simplify identification of the organic fluorides for a systematic search, is used today as the numbering system for refrigerants. -

Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards
A111D3 TTbS?? o z C/J NBS SPECIAL PUBLICATION 260-108 o ^EAU U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards Standard Reference Materials: Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards QC 100 U57 R. Mavrodineanu and T. E. Gills 260-108 1987 m he National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal i s t0 strengthen and advance the nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research to assure international competitiveness and leadership of U.S. industry, science arid technology. NBS work involves development and transfer of measurements, standards and related science and technology, in support of continually improving U.S. productivity, product quality and reliability, innovation and underlying science and engineering. The Bureau's technical work is performed by the National Measurement Laboratory, the National Engineering Laboratory, the Institute for Computer Sciences and Technology, and the Institute for Materials Science and Engineering. The National Measurement Laboratory Provides the national system of physical and chemical measurement; • Basic Standards 2 coordinates the system with measurement systems of other nations and • Radiation Research furnishes essential services leading to accurate and uniform physical and • Chemical Physics chemical measurement throughout the Nation's scientific community, • Analytical Chemistry industry, and commerce; provides advisory and research services to other Government agencies; conducts physical and chemical research; develops, produces, and distributes Standard Reference Materials; provides calibration services; and manages the National Standard Reference Data System. -

Evolution of Refrigerants Mark O
This is an open access article published under an ACS AuthorChoice License, which permits copying and redistribution of the article or any adaptations for non-commercial purposes. pubs.acs.org/jced Review (R)Evolution of Refrigerants Mark O. McLinden* and Marcia L. Huber Cite This: J. Chem. Eng. Data 2020, 65, 4176−4193 Read Online ACCESS Metrics & More Article Recommendations *sı Supporting Information ABSTRACT: As we enter the “fourth generation” of refrigerants, we consider the evolution of refrigerant molecules, the ever- changing constraints and regulations that have driven the need to consider new molecules, and the advancements in the tools and property models used to identify new molecules and design equipment using them. These separate aspects are intimately intertwined and have been in more-or-less continuous development since the earliest days of mechanical refrigeration, even if sometimes out-of-sight of the mainstream refrigeration industry. We highlight three separate, comprehensive searches for new refrigerantsin the 1920s, the 1980s, and the 2010sthat sometimes identified new molecules, but more often, validated alternatives already under consideration. A recurrent theme is that there is little that is truly new. Most of the “new” refrigerants, from R-12 in the 1930s to R- 1234yf in the early 2000s, were reported in the chemical literature decades before they were considered as refrigerants. The search for new refrigerants continued through the 1990s even as the hydrofluorocarbons (HFCs) were becoming the dominant refrigerants in commercial use. This included a return to several long-known natural refrigerants. Finally, we review the evolution of the NIST REFPROP database for the calculation of refrigerant properties. -

Finding Refrigerant Leaks with the Chempro100i
Application Note: 108 Finding Refrigerant Leaks with the ChemPro100i The ChemPro100i is a great sniffer for halocarbon refrigerants, often generically referred to as “Freon” because of its multiple sensors and broad sniffing capability. As refrigerant sniffers are only carried by a small subset of first responders, the ChemPro100i can fill the role for those that don’t have a refrigerant sniffer. As a bonus, if someone suspects that it may be a refrigerant leak, and it turns out to be something else, the wide range of detectable gases and vapors seen by the ChemPro100i means that it will most likely find the unexpected gas/vapor too. The ChemPro100i’s Sensors The ChemPro100i uses a suite of seven sensors including an aspirated Ion Mobility Spectroscopy (IMS), five metal oxide sensors and a field effect sensor to detect, characterize, and even identify, some gases and vapors. Using this suite of sensors, the ChemPro100i can find halocarbon refrigerant leaks using its “Trend” or “sniffer” screen. As one gets closer to the refrigerant leak, the trend line will Using other Sniffers for increase. This is a non-quantifiable reading Refrigerants that does not directly correlate with parts per Most dedicated refrigerant detectors use a million (ppm), but the fast response of the Metal Oxide Sensor (MOS) that is doped to ChemPro100i to refrigerants provides one with be relatively specific to the halogenated the means of quickly finding a refrigerant leak. hydrocarbons (halogens) that are the hallmark of most refrigerants. MOS sensors Increase in the Trend line leads you to the leak are non-linear and not suitable for quantification of refrigerants, but they provide sensitive and fast response to halocarbon refrigerants.