Dichlorodifluoromethane Dcf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Refrigerant Safety Refrigerant History
Refrigerant Safety The risks associated with the use of refrigerants in refrigeration and airconditioning equipment can include toxicity, flammability, asphyxiation, and physical hazards. Although refrigerants can pose one or more of these risks, system design, engineering controls, and other techniques mitigate this risk for the use of refrigerant in various types of equipment. Refrigerant History Nearly all of the historically used refrigerants were flammable, toxic, or both. Some were also highly reactive, resulting in accidents (e. g., leak, explosion) due to equipment failure, poor maintenance, or human error. The task of finding a nonflammable refrigerant with good stability was given to Thomas Midgley in 1926. With his associates Albert Leon Henne and Robert Reed McNary, Dr. Midgley observed that the refrigerants then in use comprised relatively few chemical elements, many of which were clustered in an intersecting row and column of the periodic table of elements. The element at the intersection was fluorine, known to be toxic by itself. Midgley and his collaborators felt, however, that compounds containing fluorine could be both nontoxic and nonflammable. The attention of Midgley and his associates was drawn to organic fluorides by an error in the literature that showed the boiling point for tetrafluoromethane (carbon tetrafluoride) to be high compared to those for other fluorinated compounds. The correct boiling temperature later was found to be much lower. Nevertheless, the incorrect value was in the range sought and led to evaluation of organic fluorides as candidates. The shorthand convention, later introduced to simplify identification of the organic fluorides for a systematic search, is used today as the numbering system for refrigerants. -

Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards
A111D3 TTbS?? o z C/J NBS SPECIAL PUBLICATION 260-108 o ^EAU U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards Standard Reference Materials: Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards QC 100 U57 R. Mavrodineanu and T. E. Gills 260-108 1987 m he National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal i s t0 strengthen and advance the nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research to assure international competitiveness and leadership of U.S. industry, science arid technology. NBS work involves development and transfer of measurements, standards and related science and technology, in support of continually improving U.S. productivity, product quality and reliability, innovation and underlying science and engineering. The Bureau's technical work is performed by the National Measurement Laboratory, the National Engineering Laboratory, the Institute for Computer Sciences and Technology, and the Institute for Materials Science and Engineering. The National Measurement Laboratory Provides the national system of physical and chemical measurement; • Basic Standards 2 coordinates the system with measurement systems of other nations and • Radiation Research furnishes essential services leading to accurate and uniform physical and • Chemical Physics chemical measurement throughout the Nation's scientific community, • Analytical Chemistry industry, and commerce; provides advisory and research services to other Government agencies; conducts physical and chemical research; develops, produces, and distributes Standard Reference Materials; provides calibration services; and manages the National Standard Reference Data System. -

Flammable Refrigerants Firefighter Training
Flammable refrigerants firefighter training: Hazard assessment and demonstrative testing FINAL REPORT BY: Noah L. Ryder, P. E. Stephen J. Jordan Fire & Risk Alliance, LLC. Rockville, Maryland, USA. Peter B. Sunderland, Ph.D. University of Maryland Department of Fire Protection Engineering College Park, Maryland, USA. May 2019 © 2019 Fire Protection Research Foundation 1 Batterymarch Park, Quincy, MA 02169-7417, USA Email: [email protected] | Web: nfpa.org/foundation ---page intentionally left blank--- —— Page ii —— FOREWORD The ongoing push toward sustainability of refrigeration systems will require the adoption of low global warming potential (GWP) refrigerants to meet the shift in environmental regulations. Fire safety is a lingering issue with the new age of flammable refrigerants being adopted and first responders may not be familiar with the change in material hazards or the appropriate response procedures required to safely handle these fire scenarios. This project is part of the overall two-year project with a goal to enhance firefighter safety and reduce potential injury by providing training on the hazards from appliances with flammable refrigerants. It will document the information about flammable refrigerants technologies and the hazards to emergency responders and develop interactive training modules to transfer the knowledge to the fire service. This report is focused to develop material documenting the hazards associated with flammable refrigerant technologies and the risks posed to the first responders. The material documentation included a literature review, Task 1, identifying baseline information on flammable refrigerants, their existing usage and implementation into products, potential integration into future technologies, and finally any existing guidance and best practices on response and tactics. -
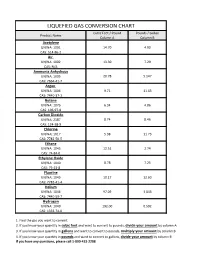
Liquefied Gas Conversion Chart
LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Acetylene UN/NA: 1001 14.70 4.90 CAS: 514-86-2 Air UN/NA: 1002 13.30 7.29 CAS: N/A Ammonia Anhydrous UN/NA: 1005 20.78 5.147 CAS: 7664-41-7 Argon UN/NA: 1006 9.71 11.63 CAS: 7440-37-1 Butane UN/NA: 1075 6.34 4.86 CAS: 106-97-8 Carbon Dioxide UN/NA: 2187 8.74 8.46 CAS: 124-38-9 Chlorine UN/NA: 1017 5.38 11.73 CAS: 7782-50-5 Ethane UN/NA: 1045 12.51 2.74 CAS: 74-84-0 Ethylene Oxide UN/NA: 1040 8.78 7.25 CAS: 75-21-8 Fluorine UN/NA: 1045 10.17 12.60 CAS: 7782-41-4 Helium UN/NA: 1046 97.09 1.043 CAS: 7440-59-7 Hydrogen UN/NA: 1049 192.00 0.592 CAS: 1333-74-0 1. Find the gas you want to convert. 2. If you know your quantity in cubic feet and want to convert to pounds, divide your amount by column A 3. If you know your quantity in gallons and want to convert to pounds, multiply your amount by column B 4. If you know your quantity in pounds and want to convert to gallons, divide your amount by column B If you have any questions, please call 1-800-433-2288 LIQUEFIED GAS CONVERSION CHART Cubic Feet / Pound Pounds / Gallon Product Name Column A Column B Hydrogen Chloride UN/NA: 1050 10.60 8.35 CAS: 7647-01-0 Krypton UN/NA: 1056 4.60 20.15 CAS: 7439-90-9 Methane UN/NA: 1971 23.61 3.55 CAS: 74-82-8 Methyl Bromide UN/NA: 1062 4.03 5.37 CAS: 74-83-9 Neon UN/NA: 1065 19.18 10.07 CAS: 7440-01-9 Mapp Gas UN/NA: 1060 9.20 4.80 CAS: N/A Nitrogen UN/NA: 1066 13.89 6.75 CAS: 7727-37-9 Nitrous Oxide UN/NA: 1070 8.73 6.45 CAS: 10024-97-2 Oxygen UN/NA: 1072 12.05 9.52 CAS: 7782-44-7 Propane UN/NA: 1075 8.45 4.22 CAS: 74-98-6 Sulfur Dioxide UN/NA: 1079 5.94 12.0 CAS: 7446-09-5 Xenon UN/NA: 2036 2.93 25.51 CAS: 7440-63-3 1. -

Product Stewardship Summary Carbon Tetrachloride
Product Stewardship Summary Carbon Tetrachloride May 31, 2017 version Summary This Product Stewardship Summary is intended to give general information about Carbon Tetrachloride. It is not intended to provide an in-depth discussion of all health and safety information about the product or to replace any required regulatory communications. Carbon tetrachloride is a colorless liquid with a sweet smell that can be detected at low levels; however, odor is not a reliable indicator that occupational exposure levels have not been exceeded. Its chemical formula is CC14. Most emissive uses of carbon tetrachloride, a “Class I” ozone-depleting substance (ODS), have been phased out under the Montreal Protocol on Substances that Deplete the Ozone Layer (Montreal Protocol). Certain industrial uses (e.g. feedstock/intermediate, essential laboratory and analytical procedures, and process agent) are allowed under the Montreal Protocol. 1. Chemical Identity Name: carbon tetrachloride Synonyms: tetrachloromethane, methane tetrachloride, perchloromethane, benzinoform Chemical Abstracts Service (CAS) number: 56-23-5 Chemical Formula: CC14 Molecular Weight: 153.8 Carbon tetrachloride is a colorless liquid. It has a sweet odor, and is practically nonflammable at ambient temperatures. 2. Production Carbon tetrachloride can be manufactured by the chlorination of methyl chloride according to the following reaction: CH3C1+ 3C12 —> CC14 + 3HC1 Carbon tetrachloride can also be formed as a byproduct of the manufacture of chloromethanes or perchloroethylene. OxyChem manufactures carbon tetrachloride at facilities in Geismar, Louisiana and Wichita, Kansas. Page 1 of 7 3. Uses The use of carbon tetrachloride in consumer products is banned by the Consumer Product Safety Commission (CPSC), under the Federal Hazardous Substance Act (FHSA) 16 CFR 1500.17. -

Toxicological Profile for Carbon Tetrachloride
CARBON TETRACHLORIDE 175 5. PRODUCTION, IMPORT/EXPORT, USE, AND DISPOSAL 5.1 PRODUCTION Carbon tetrachloride is produced by exhaustive chlorination of a variety of low molecular weight hydrocarbons such as carbon disulfide, methane, ethane, propane, and ethylene dichloride (HSDB 2004). It is also produced by thermal chlorination of methyl chloride (HSDB 2004). Carbon tetrachloride is a feedstock for chlorofluorocarbon gases, such as dichlorodifluoromethane (F-12) and trichlorofluoro methane (F-11), which were used as aerosol propellants in the 1950s and 1960s (Holbrook 1991). Following this, the growth rate for the production of carbon tetrachloride averaged 10.7% per year from 1960 to 1970 (Holbrook 1991). This rate slowed to 7.2% per year from 1970 to 1974, when the production of this chemical was at its peak, as other forms of propellants became commercially available (Anonymous 1981; Holbrook 1991). The FDA banned the sale of carbon tetrachloride in any product used in the home and the EPA regulated the use of chlorofluorocarbon gases as aerosols or propellants. Since then, production of carbon tetrachloride has declined at approximately 8% a year from 1974 to 1994 (Anonymous 1995; Holbrook 1991). Carbon tetrachloride is currently manufactured in the United States by Vulcan Materials Company at two plants: Geismar, Louisiana and Wichita, Kansas, with a combined 130 million pound capacity (HSDB 2004; SRI 2004). It should be noted, however, that these capacities are flexible, since other chlorinated solvents are made using the same equipment (SRI 2004). This recent decline in production is due to the adoption of an international agreement (the Montreal Protocol) to reduce environmental concentrations of ozone-depleting chemicals (including carbon tetrachloride), and to the provisions of Title VI of the Clean Air Act Amendments of 1990 addressing these chemicals. -

Dichlorodifluoromethane 1018
DICHLORODIFLUOROMETHANE 1018 CCl2F2 MW: 120.91 CAS: 75-71-8 RTECS: PA8200000 METHOD: 1018, Issue 2 EVALUATION: FULL Issue 1: 15 August 1987 Issue 2: 15 August 1994 OSHA : 1000 ppm PROPERTIES: gas; BP •29.8 °C; MP •158 °C: NIOSH: 1000 ppm d 1.35 @ 21.1 °C; nonflammable ACGIH: 1000 ppm (1 ppm = 4.95 mg/m 3 @ NTP) SYNONYMS: difluorodichloromethane; Refrigerant 12 SAMPLING MEASUREMENT SAMPLER: SOLID SORBENT TUBES TECHNIQUE: GAS CHROMATOGRAPHY, FID (two coconut shell charcoal tubes in series, 400/200 mg and 100/50 mg) ANALYTE: dichlorodifluoromethane FLOW RATE: 0.01 to 0.05 L/min DESORPTION: 20 mL methylene chloride VOL-MIN: 1 L @ 1000 ppm INJECTION -MAX: 4 L VOLUME: 1 µL SHIPMENT: refrigerated TEMPERATURE-INJECTOR: 200 °C -DETECTOR: 260 °C SAMPLE -COLUMN: 35 °C for 3 min, STABILITY: at least 7 days @ •10 °C 15 °C/min to 75 °C, hold 6 min BLANKS: 2 to 10 field blanks per set CARRIER GAS: He, 1.5 mL/min COLUMN: DB-1 fused silica capillary, 30 m x 0.32-mm ID, 1-µm film thickness or equivalent ACCURACY CALIBRATION: standard solutions of analyte in RANGE STUDIED: 2940 to 10,500 mg/m 3 [1] methylene chloride BIAS: • 1.8% RANGE: 5 to 30 mg per sample [1] ˆ OVERALL PRECISION (S rT): 0.063 [1] ESTIMATED LOD: 0.03 mg per sample [1] ACCURACY: ± 12.8% PRECISION (S r): 0.035 @ 7.4-30 mg per sample [1] APPLICABILITY: The working ranges are 1000 to 6000 ppm dichlorodifluoromethane (5000 to 30,000 mg/m 3); 700 to 5700 ppm 1,2-dichlorotetrafluoromethane (5000 to 40,000 mg/m 3); and 300 to 4000 ppm chlorodifluoromethane (1000 to 14,000 mg/m3) for a 1-L air sample. -

SAFETY DATA SHEET Halocarbon R-12 (Dichlorodifluoromethane)
SAFETY DATA SHEET Halocarbon R-12 (Dichlorodifluoromethane) Section 1. Identification GHS product identifier : Halocarbon R-12 (Dichlorodifluoromethane) Chemical name : dichlorodifluoromethane Other means of : ASPEN R-12, Methane, dichlorodifluoro-; Refrigerant 12; Propellant 12; Halon 122; identification Genetron 12; Freon 12; Fluorocarbon 12; Difluorodichloromethane; DICHLORODIFLUOROMETHANE (FC 12); CFC-12 Product type : Gas. Product use : Synthetic/Analytical chemistry. Synonym : ASPEN R-12, Methane, dichlorodifluoro-; Refrigerant 12; Propellant 12; Halon 122; Genetron 12; Freon 12; Fluorocarbon 12; Difluorodichloromethane; DICHLORODIFLUOROMETHANE (FC 12); CFC-12 SDS # : 001018 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture HAZARDOUS TO THE OZONE LAYER - Category 1 GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Harms public health and the environment by destroying ozone in the upper atmosphere. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. -

Safety Data Sheet
SAFETY DATA SHEET Flammable Liquefied Gas Mixture: 1,1,1,2-Tetrafluoropropane \ 1,1,1,2, 2-Pentafluoropropane \ 2-Chloro-3,3,3-Trifluoropropene \ 2,3,3,3-Tetrafluoropropene \ 3,3,3-Trifluoropropene \ 3,3,3-Trifluoropropyne \ Cis-1-Chloro-2-Fluoro-Ethylene \ Methyl Chloride \ R12-Dichlorodifluoromethane \ R124-Chlorotetrafluoroethane \ R134A-Tetrafluoroethane \ R218-Octafluoropropane \ R244Bb- Chlorotetrafluoropropane \ R244Cc-Chlorotetrafluoropropane \ Trans-1-Chloro- 2-Fluoroethylene \ Trans-1,3,3,3-Tetrafluoropropene \ Vinyl Chloride Section 1. Identification GHS product identifier : Flammable Liquefied Gas Mixture: 1,1,1,2-Tetrafluoropropane \ 1,1,1,2, 2-Pentafluoropropane \ 2-Chloro-3,3,3-Trifluoropropene \ 2,3,3,3-Tetrafluoropropene \ 3, 3,3-Trifluoropropene \ 3,3,3-Trifluoropropyne \ Cis-1-Chloro-2-Fluoro-Ethylene \ Methyl Chloride \ R12-Dichlorodifluoromethane \ R124-Chlorotetrafluoroethane \ R134A- Tetrafluoroethane \ R218-Octafluoropropane \ R244Bb-Chlorotetrafluoropropane \ R244Cc-Chlorotetrafluoropropane \ Trans-1-Chloro-2-Fluoroethylene \ Trans-1,3,3, 3-Tetrafluoropropene \ Vinyl Chloride Other means of : Not available. identification Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. SDS # : 022757 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : FLAMMABLE GASES - Category 1 substance or mixture GASES UNDER PRESSURE - Liquefied gas GHS label elements Hazard pictograms : Signal word : Danger Hazard statements : Extremely flammable gas. May form explosive mixtures with air. Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. -

METHANE, Cfcs, and OTHER GREENHOUSE GASES Donald R
Chapter 17 METHANE, CFCs, AND OTHER GREENHOUSE GASES Donald R. Blake Our planet is continuously bathed in solar radiation. Although we who are confined to a fixed location on the globe experience day and night, the earth does not. It is always day in the sense that the sun is shining on half of the globe. Much of the incoming solar radiation, about 30%, is scattered back to space by clouds, atmospheric gases and particles, and objects on the earth. The remaining 70% is, therefore, absorbed mostly at the earth's surface. This absorbed radiation gives up its energy to whatever absorbed it, thereby causing its temperature to increase. Be- Testimony given in hearings before the Committee on Energy and Natural Resources, U.S. Senate, One-hundredth Congress, first session, on the Greenhouse Effect and Global Climate Change, 9-10 November 1987 . _ The original publication of this paper provided an illustration that has not been included here. This illustration depicts the monthly means and variances of the trace gases tri chlorofluoromethane (CFC- I I, or CFCl5) , dichlorodifluoromethane (CFC-I 2, CF2Cl2), methyl chloroform (CH3CCl3), carbon tetrachloride (CCl4 ) , and nitrous oxide (N20) as measured at the Barbados station of the Global Atmospheric Gases Experiment. 248 METHANE, CFCs, AND OTHER GREENHOUSE GASES 249 cause solar radiation is absorbed continuously by the earth, it might be supposed that its temperature should continue to increase. It does not, of course, because the earth also emits radiation, the spectral distribution of which is quite different from that of the incoming solar radiation. The higher the earth's temperature, the more infrared radiation it emits. -

Carbon Tetrachloride
frodeFebruary 2017 Office of Chemical Safety and Pollution Prevention U.S. EPA Preliminary Information on Manufacturing, Processing, Distribution, Use, and Disposal: Carbon Tetrachloride CASRN: 56-23-5 February 2017 Support document for Docket EPA-HQ-OPPT-2016-0733 Page 1 of 21 This document provides a preliminary public summary of available information collected by EPA’s Office of Pollution Prevention and Toxics (OPPT) in the Office of Chemical Safety and Pollution Prevention (OCSPP) on the manufacturing (including importing), processing, distribution in commerce, use, and disposal of this chemical. This is based on existing data available to EPA, including information collected under the Chemical Data Reporting rule, Toxics Release Inventory, information from other Agency databases, other U.S. Government agencies, publicly available information from states, and a review of published literature. In addition, the document includes information reported to EPA by producers and users of the chemical in the United States and in other countries. This preliminary use information and any additional use information received in the docket by March 15, 2017 will inform efforts to develop the scope of the chemical risk evaluation required under section 6(b)(4) of the Toxic Substances Control Act, and will inform any risk management efforts following risk evaluation. Mention of trade names in this document does not constitute endorsement by EPA. To verify products or articles containing this chemical currently in commerce, EPA has identified several examples. Any lists are provided for informational purposes only. EPA and its employees do not endorse any of the products or companies. This document does not contain confidential business information (CBI). -

Classification of Refrigerants
INSTITUT INTERNATIONAL INTERNATIONAL INSTITUTE DU FROID OF REFRIGERATION Organisation intergouvernement ale Intergovernmental organization pour le développement du froid for the development of refrigeration 177, boulevard Malesherbes, 75017 PARIS, France - Tél. 33-(0)1 42 27 32 35 - Fax 33-(0)1 47 63 17 98 - E-mail : [email protected] - Web : www.iifiir.org Classification of refrigerants This text is based on the American standard ANSI/ASHRAE 34 published in 2001 and entitled “Designation and Safety Classification of Refrigerants”. This classification makes it possible to designate all refrigerants used in a clear and internationally recognized manner by classifying them according to their chemical composition. 1 Numbering of Refrigerants An identifying number shall be assigned to each refrigerant. It consists of a prefix made up of letters and a suffix made up of digits. 1.1 Prefixes The prefix is composed of the letter R (for refrigerant). Examples: R22, R134a, R600a, R717 Sometimes, the letter C is used in the prefix to denote carbon, preceded by B, C or F (or a combination of these letters in the same order) to indicate the presence of bromine, chlorine or fluorine. Compounds containing hydrogen must be preceded by the letter H. Examples: HCFC22, HFC134a These prefixes must only be used in non-technical publications. Note: the name of the brand or of the manufacturer is also used sometimes; these names must not be used in official documents (identification labels, etc.). 1.2 Suffixes • Hydrocarbons and derivatives The first digit on the right (units) is the number of fluorine (F) atoms. The second digit on the right (tens) is one more than the number of hydrogen (H) atoms.