SW-846 Update VI 8260D - 1 Revision 4 June 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bromomethane CAS #: 74-83-9 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015
CHEMICAL UPDATE WORKSHEET Chemical Name: Bromomethane CAS #: 74-83-9 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015 (A) Chemical-Physical Properties Part 201 Value Updated Value Reference Source Comments Molecular Weight (g/mol) 94.94 94.94 EPI EXP Physical State at ambient temp Liquid Gas MDEQ Melting Point (˚C) 179 -93.70 EPI EXP Boiling Point (˚C) 3.5 3.50 EPI EXP Solubility (ug/L) 1.45E+7 1.52E+07 EPI EXP Vapor Pressure (mmHg at 25˚C) 1672 1.62E+03 EPI EXP HLC (atm-m³/mol at 25˚C) 1.42E-2 7.34E-03 EPI EXP Log Kow (log P; octanol-water) 1.18 1.19 EPI EXP Koc (organic carbon; L/Kg) 14.5 13.22 EPI EST Ionizing Koc (L/kg) NR NA NA Diffusivity in Air (Di; cm2/s) 0.08 1.00E-01 W9 EST Diffusivity in Water (Dw; cm2/s) 8.0E-6 1.3468E-05 W9 EST CHEMICAL UPDATE WORKSHEET Bromomethane (74-83-9) Part 201 Value Updated Value Reference Source Comments Soil Water Partition Coefficient NR NR NA NA (Kd; inorganics) Flash Point (˚C) NA 194 PC EXP Lower Explosivity Level (LEL; 0.1 0.1 CRC EXP unit less) Critical Temperature (K) 467.00 EPA2004 EXP Enthalpy of Vaporization 5.71E+03 EPA2004 EXP (cal/mol) Density (g/mL, g/cm3) 1.6755 CRC EXP EMSOFT Flux Residential 2 m 2.69E-05 2.80E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Residential 5 m 6.53E-05 6.86E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Nonresidential 2 m 3.83E-05 4.47E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Nonresidential 5 m 9.24E-05 1.09E-04 EMSOFT EST (mg/day/cm2) 2 CHEMICAL UPDATE WORKSHEET Bromomethane (74-83-9) (B) Toxicity Values/Benchmarks Source/Reference/ Comments/Notes Part 201 Value Updated Value Date /Issues Reference Dose 1.4E-3 2.0E-2 OPP, 2013 (RfD) (mg/kg/day) Rat subchronic Tier 1 Source: Complete gavage study EPA-OPP: (Danse et al., Basis: OPP is the more current than IRIS, PPRTV and ATSDR. -

SAFETY DATA SHEET Bromomethane (R40 B1) SECTION 1
SAFETY DATA SHEET Bromomethane (R40 B1) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021848 Last revised date: 02.02.2017 1/17 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Product name: Bromomethane (R40 B1) Additional identification Chemical name: Bromomethane Chemical formula: CH3Br INDEX No. 602-002-00-2 CAS-No. 74-83-9 EC No. 200-813-2 REACH Registration No. Not available. 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Industrial and professional. Perform risk assessment prior to use. Using gas alone or in mixtures for the calibration of analysis equipment. Using gas as feedstock in chemical processes. Formulation of mixtures with gas in pressure receptacles. Uses advised against Consumer use. 1.3 Details of the supplier of the safety data sheet Supplier Linde Gas GmbH Telephone: +43 50 4273 Carl-von-Linde-Platz 1 A-4651 Stadl-Paura E-mail: [email protected] 1.4 Emergency telephone number: Emergency number Linde: + 43 50 4273 (during business hours), Poisoning Information Center: +43 1 406 43 43 SDS_AT - 000010021848 SAFETY DATA SHEET Bromomethane (R40 B1) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021848 Last revised date: 02.02.2017 2/17 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Directive 67/548/EEC or 1999/45/EC as amended. T; R23/25 Xi; R36/37/38 Xn; R48/20 Muta. 3; R68 N; R50 N; R59 The full text for all R-phrases is displayed in section 16. -

RR Program's RCL Spreadsheet Update
RR Program’s RCL Spreadsheet Update March 2017 RR Program RCL Spreadsheet Update DNR-RR-052e The Wisconsin DNR Remediation and Redevelopment Program (RR) has updated the numerical soil standards in the August 2015 DNR-RR- 052b RR spreadsheet of residual contaminant levels (RCLs). The RCLs were determined using the U.S. EPA RSL web- calculator by accepting EPA exposure defaults, with the exception of using Chicago, IL, for the climatic zone. This documentThe U.S. provides EPA updateda summary its Regionalof changes Screening to the direct-contact Level (RSL) RCLs website (DC-RCLs) in June that2015. are To now reflect in the that March 2017 spreadsheet.update, the The Wisconsin last page ofDNR this updated document the has numerical the EPA exposuresoil standards, parameter or residual values usedcontaminant in the RCL levels calculations. (RCLs), in the Remediation and Redevelopment program’s spreadsheet of RCLs. This document The providesU.S. EPA a RSL summary web-calculator of the updates has been incorporated recently updated in the Julyso that 2015 the spreadsheet.most up-to-date There toxicity were values no changes for chemi - cals madewere certainlyto the groundwater used in the RCLs,RCL calculations. but there are However, many changes it is important in the industrial to note that and the non-industrial web-calculator direct is only a subpartcontact of the (DC) full RCLsEPA RSL worksheets. webpage, Tables and that 1 andthe other 2 of thissubparts document that will summarize have important the DC-RCL explanatory changes text, generic tablesfrom and the references previous have spreadsheet yet to be (Januaryupdated. -

A High Volume Sampling System for Isotope Determination of Volatile Halocarbons and Hydrocarbons
Atmos. Meas. Tech., 4, 2073–2086, 2011 www.atmos-meas-tech.net/4/2073/2011/ Atmospheric doi:10.5194/amt-4-2073-2011 Measurement © Author(s) 2011. CC Attribution 3.0 License. Techniques A high volume sampling system for isotope determination of volatile halocarbons and hydrocarbons E. Bahlmann, I. Weinberg, R. Seifert, C. Tubbesing, and W. Michaelis Institute for Biogeochemistry and Marine Chemistry, Hamburg, Germany Received: 15 March 2011 – Published in Atmos. Meas. Tech. Discuss.: 8 April 2011 Revised: 31 August 2011 – Accepted: 7 September 2011 – Published: 4 October 2011 Abstract. The isotopic composition of volatile organic com- 1 Introduction pounds (VOCs) can provide valuable information on their sources and fate not deducible from mixing ratios alone. Compound specific isotope ratio mass spectrometry In particular the reported carbon stable isotope ratios of (CSIRMS) of non methane volatile organic compounds chloromethane and bromomethane from different sources (NMVOCs) emerged as a powerful tool to distinguish dif- 13 cover a δ C-range of almost 100 ‰ making isotope ra- ferent sources and to provide information on sinks (Rudolph tios a very promising tool for studying the biogeochemistry et al., 1997; Rudolph and Czuba, 2000; Tsunogai et al., of these compounds. So far, the determination of the iso- 1999; Bill et al., 2002; Thompson et al., 2002; Goldstein and topic composition of C1 and C2 halocarbons others than Shaw, 2003 and references therein; Archbold et al., 2005; chloromethane is hampered by their low mixing ratios. Redeker -

Argonne Report.Pdf
CONTENTS NOTATION ........................................................................................................................... xi ABSTRACT ........................................................................................................................... 1 1 INTRODUCTION ........................................................................................................... 5 1.1 Overview of the Emergency Response Guidebook ................................................ 5 1.2 Organization of this Report ..................................................................................... 7 2 GENERAL METHODOLOGY ....................................................................................... 9 2.1 TIH List ................................................................................................................... 10 2.1.1 Background ................................................................................................. 10 2.1.2 Changes in the TIH List for the ERG2012 ................................................. 11 2.2 Shipment and Release Scenarios ............................................................................ 11 2.2.1 Shipment Profiles ........................................................................................ 12 2.2.2 Treatment of Chemical Agents ................................................................... 14 2.3 Generics, Mixtures, and Solutions .......................................................................... 17 2.4 Analysis of Water-Reactive -

Measurement Technique for the Determination of Photolyzable
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 102, NO. D13, PAGES 15,999-16,004,JULY 20, 1997 Measurement techniquefor the determination of photolyzable chlorine and bromine in the atmosphere G. A. Impey,P. B. Shepson,• D. R. Hastie,L. A. Bartie• Departmentof Chemistryand Centre for AtmosphericChemistry, York University,Toronto, Ontario, Canada Abstract. A techniquehas been developed to enablemeasurement of photolyzablechlorine and bromineat tracelevels in the troposphere.In thismethod, ambient air is drawnt•ough a cylindricalflow cell, whichis irradiatedwith a Xe arc lamp. In the reactionvessel of the photoactivehalogen detector (PHD), photolyrically active molecules Clp (including C12, HOC1, C1NO,C1NO2, and C1ONO2) and Brp (including Br2, HOBr, BrNO, BrNO2, and BrONO2) are photolyzed,and the halogenatoms produced react with properieto form stablehalogenated products.These products are thensampled and subsequently separated and detected by gas chromatography.The systemis calibratedusing low concentrationmixtures of C12and Br2 in air from commerciallyavailable permeation sources. We obtaineddetection limits of 4 pptv and 9 pptv as Br2 andC12, respectively, for 36 L samples. 1. Introduction (or C12)in the Arctic, largely as a result of the lack of suitable analyticalmethodologies. This paperreports the developmentof The episodicdestruction of groundlevel ozonein the Arctic at a measurementtechnique for the determinationof rapidly sunriseis a phenomenonthat hasbeen observed for many years. photolyzingchlorine (referred to hereas Clp) and bromine (Brp) With the onsetof polar sunrise,ozone levels are often observed speciesat pansper trillion by volume(pptv) mixingratios in the to drop from a backgroundconcentration of •40 ppbv to almost atmosphere.Impey et al. [this issue]discuss the resultsobserved zero on a timescaleof a day or less [Barrie et al., 1988] for from a field studyconducted in the Canadianhigh Arctic at Alert, periodsof 1-10 days. -
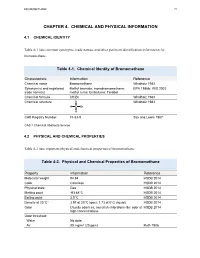
Toxicological Profile for Bromomethane
BROMOMETHANE 72 CHAPTER 4. CHEMICAL AND PHYSICAL INFORMATION 4.1 CHEMICAL IDENTITY Table 4-1 lists common synonyms, trade names, and other pertinent identification information for bromomethane. Table 4-1. Chemical Identity of Bromomethane Characteristic Information Reference Chemical name Bromomethane Windholz 1983 Synonym(s) and registered Methyl bromide; monobromomethane; EPA 1986b; IRIS 2002 trade name(s) methyl fume; Embafume; Terabol Chemical formula CH3Br Windholz 1983 Chemical structure H Windholz 1983 H C Br H CAS Registry Number 74-83-9 Sax and Lewis 1987 CAS = Chemical Abstracts Service 4.2 PHYSICAL AND CHEMICAL PROPERTIES Table 4-2 lists important physical and chemical properties of bromomethane. Table 4-2. Physical and Chemical Properties of Bromomethane Property Information Reference Molecular weight 94.94 HSDB 2014 Color Colorless HSDB 2014 Physical state Gas HSDB 2014 Melting point -93.68°C HSDB 2014 Boiling point 3.5°C HSDB 2014 Density at 20°Ca 3.97 at 20°C (gas); 1.73 at 0°C (liquid) HSDB 2014 Odor Usually odorless; sweetish chloroform-like odor at HSDB 2014 high concentrations Odor threshold: Water No data Air 80 mg/m3 (20 ppm) Ruth 1986 BROMOMETHANE 73 4. CHEMICAL AND PHYSICAL INFORMATION Table 4-2. Physical and Chemical Properties of Bromomethane Solubility: Water at 20°C 15.2 g/L at 25°C HSDB 2014 18.5 g/L at 20°C 13.4 g/L at 25°C Organic solvents Readily soluble in lower alcohols, ethers, esters, HSDB 2014 ketones, halogenated hydrocarbons, aromatic hydrocarbons, and carbon disulfide; freely soluble in benzene, carbon tetrachloride, and carbon disulfide; miscible in ethanol and chloroform Partition coefficients: Log Kow 1.19 HSDB 2014 Log Koc 0.95–1.3 Yates et al. -
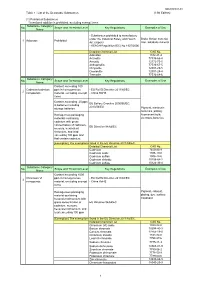
Table 1 : List of the Declarable Substances (1) Prohibited
DQ2000001-03 Table 1 : List of the Declarable Substances (13th Edition) (1) Prohibited Substances *Intentional addition is prohibited, excluding exempt items Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name - Substances prohibited to manufacture under the Industrial Safety and Health Brake friction material, 1 Asbestos Prohibited Act (Japan) filler, adiabatic material - REACH Regulation (EC) No 1907/2006 Detailed Chemical List CAS No. Asbestos 1332-21-4 Actinolite 77536-66-4 Amosite 12172-73-5 Anthophyllite 77536-67-5 Chrysotile 12001-29-5 Crocidolite 12001-28-4 Tremolite 77536-68-6 Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name Content exceeding 100 Cadmium/cadmium ppm in homogeneous - EU RoHS Directive 2011/65/EC 2 compounds material, excluding exempt - China RoHS items Content exceeding 20 ppm EU Battery Directive 2006/66/EC, in batteries including 2013/56/EU storage batteries Pigment, electronic materials, plating, Homogenous packaging fluorescent bulb, materials containing electrode,batteries cadmium with gross concentration of cadmium, EU Directive 94/62/EC mercury, hexavalent chromium, and lead exceeding 100 ppm and that contain cadmium [Exemption] The exemptions listed in the EU Directive 2011/65/EC. Detailed Chemical List CAS No. Cadmium 7440-43-9 Cadmium oxide 1306-19-0 Cadmium sulfide 1306-23-6 Cadmium chloride 10108-64-2 Cadmium sulfate 10124-36-4 Substance Category/ No. Scope and Threshold Level Key Regulations Examples of Use Name Content exceeding 1000 Chromium VI ppm in homogeneous - EU RoHS Directive 2011/65/EC 3 compounds material, excluding exempt - China RoHS items Homogenous packaging Pigment, catalyst, material containing plating, dye, surface hexavalent chromium with treatment gross concentration of EU Directive 94/62/EC cadmium, mercury, hexavalent chromium, and lead exceeding 100 ppm [Exemption] The exemptions listed in the EU Directive 2011/65/EC. -

Acutely Hazardous Waste List
ACUTELY HAZARDOUS WASTE P011 1303–28–2 Arsenic pentoxide P012 1327–53–3 Arsenic trioxide The following materials, when a waste, are specifically listed in P038 692–42–2 Arsine, diethyl- 40 CFR 261.33 as Acutely Hazardous Wastes, when they are the only active ingredient, and are unused/unaltered. Also, P036 696–28–6 Arsonous dichloride, phenyl- certain solvent mixtures (of at least 10%) containing dioxin are P054 151–56–4 Aziridine Acutely hazardous wastes. P067 75–55–8 Aziridine, 2-methyl- P013 542–62–1 Barium cyanide The primary hazardous property(ies) of these materials are P024 106–47–8 Benzenamine, 4-chloro- indicated by the letters T (Toxicity), R (Reactivity), I (Ignitability) and C (Corrosivity). Absence of a letter indicates that the P077 100–01–6 Benzenamine, 4-nitro- compound is only listed for toxicity. P028 100–44–7 Benzene, (chloromethyl)- 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51–43–4 The list was last updated on 10/30/08. (R)- P046 122–09–8 Benzeneethanamine, alpha,alpha-dimethyl- Haz P014 108–98–5 Benzenethiol waste CAS Material 7-Benzofuranol, 2,3-dihydro-2,2-dimethyl-, No. P127 1563–66–2 methylcarbamate. P023 107–20–0 Acetaldehyde, chloro- Benzoic acid, 2-hydroxy-, compd. with (3aS-cis)- P002 591–08–2 Acetamide, N-(aminothioxomethyl)- P188 57–64–7 1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethylpyrrolo[2,3- P057 640–19–7 Acetamide, 2-fluoro- b]indol-5-yl methylcarbamate ester (1:1). P058 62–74–8 Acetic acid, fluoro-, sodium salt 2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1- 1 P002 591–08–2 1-Acetyl-2-thiourea P001 81–81–2 phenylbutyl)-, & salts, when present at concentrations greater than 0.3% P003 107–02–8 Acrolein P028 100–44–7 Benzyl chloride P070 116–06–3 Aldicarb P015 7440–41–7 Beryllium powder P203 1646–88–4 Aldicarb sulfone. -

Chemical Warfare Agents
Manuscript for Kirk-Othmer Encyclopedia of Chemical Technology August 2019 CHEMICAL WARFARE AGENTS This is the pre-print manuscript of an article published in the Kirk-Othmer Encyclopedia of Chemical Technology: https://onlinelibrary.wiley.com/doi/book/10.1002/0471238961 The published version of the article is available at the Wiley website: https://onlinelibrary.wiley.com/doi/10.1002/0471238961.0308051308011818.a01.pub3 How to cite: Costanzi, S. (2020). Chemical Warfare Agents. In Kirk‐Othmer Encyclopedia of Chemical Technology, (Ed.). doi:10.1002/0471238961.0308051308011818.a01.pub3 Stefano Costanzi Department of Chemistry and Center for Behavioral Neuroscience American University, Washington, D.C. [email protected] Chemical weapons are weapons that exploit the toxicity of chemicals to bring about death or harm. The toxic chemicals on which chemical weapons are based are known as chemical warfare agents. The elimination of this entire category of weapons is the aim of the Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction, also known as Chemical Weapons Convention or CWC, which was opened for signature in 1993 and entered into force in 1997. Administered and implemented by the Hague- based Organisation for the Prohibition of Chemical Weapons (OPCW), the CWC is an international treaty that enjoys almost universal embracement, having been ratified or acceded by 193 States Parties. Importantly, the CWC poses a complete and absolute ban on chemical weapons, mandating State Parties to renounce “(a) to develop, produce, otherwise acquire, stockpile or retain chemical weapons, or transfer, directly or indirectly, chemical weapons to anyone; (b) to use chemical weapons; (c) to engage in any military preparations to use chemical weapons; (d) to assist, encourage or induce, in any way, anyone to engage in any activity prohibited to a State Party” under the Convention (CWC Article II, Paragraph 1) (1-3). -

Table of Water-Reactive Materials Which Produce Toxic Gases
TABLE OF WATER-REACTIVE MATERIALS WHICH PRODUCE TOXIC GASES Materials Which Produce Large Amounts of Toxic-by-Inhalation (TIH) Gas(es) When Spilled in Water ID Guide TIH Gas(es) No. No. Name of Material Produced 1162 155 Dimethyldichlorosilane HCl 1196 155 Ethyltrichlorosilane HCl 1242 139 Methyldichlorosilane HCl 1250 155 Methyltrichlorosilane HCl 1295 139 Trichlorosilane HCl 1298 155 Trimethylchlorosilane HCl 1305 155P Vinyltrichlorosilane HCl 1305 155P Vinyltrichlorosilane, inhibited HCl 1305 155P Vinyltrichlorosilane, stabilized HCl 1340 139 Phosphorus pentasulfide, free from yellow and white Phosphorus H2S 1340 139 Phosphorus pentasulphide, free from yellow and white Phosphorus H2S 1360 139 Calcium phosphide PH3 1384 135 Sodium dithionite H 2SSO2 1384 135 Sodium hydrosulfite H2SSO2 1384 135 Sodium hydrosulphite H2SSO2 1397 139 Aluminum phosphide PH3 1412 139 Lithium amide NH 3 1419 139 Magnesium aluminum phosphide PH3 1432 139 Sodium phosphide PH3 1541 155 Acetone cyanohydrin, stabilized HCN 1680 157 Potassium cyanide HCN 1680 157 Potassium cyanide, solid HCN 1689 157 Sodium cyanide HCN 1689 157 Sodium cyanide, solid HCN Chemical Symbols for TIH Gases: Br2 Bromine HF Hydrogen fluoride PH3 Phosphine Cl2 Chlorine HI Hydrogen iodide SO2 Sulfur dioxide HBr Hydrogen bromide H2S Hydrogen sulfide SO2 Sulphur dioxide SO Sulfur trioxide HCl Hydrogen chloride H2S Hydrogen sulphide 3 SO Sulphur trioxide HCN Hydrogen cyanide NH3 Ammonia 3 Page 344 Use this list only when material is spilled in water. TABLE OF INITIAL ISOLATION AND PROTECTIVE ACTION DISTANCES Page 306 SMALL SPILLS LARGE SPILLS (From a small package or small leak from a large package) (From a large package or from many small packages) First Then First Then ISOLATE PROTECT ISOLATE PROTECT in all Directions persons Downwind during- in all Directions persons Downwind during- ID DAY NIGHT DAY NIGHT No. -

SUMMARY of PARTICULARLY HAZARDOUS SUBSTANCES (By
SUMMARY OF PARTICULARLY HAZARDOUS SUBSTANCES (by alpha) Key: SC -- Select Carcinogens RT -- Reproductive Toxins AT -- Acute Toxins SA -- Readily Absorbed Through the Skin DHS -- Chemicals of Interest Revised: 11/2012 ________________________________________________________ ___________ _ _ _ _ _ _ _ _ _ _ _ ||| | | | CHEMICAL NAME CAS # |SC|RT| AT | SA |DHS| ________________________________________________________ ___________ | _ | _ | _ | _ | __ | | | | | | | 2,4,5-T 000093-76-5 | | x | | x | | ABRIN 001393-62-0 | | | x | | | ACETALDEHYDE 000075-07-0 | x | | | | | ACETAMIDE 000060-35-5 | x | | | | | ACETOHYDROXAMIC ACID 000546-88-3 ||x| | x | | ACETONE CYANOHYDRIN, STABILIZED 000075-86-5 | | | x | | x | ACETYLAMINOFLUORENE,2- 000053-96-3 | x | | | | | ACID MIST, STRONG INORGANIC 000000-00-0 | x | | | | | ACROLEIN 000107-02-8 | | x | x | x | | ACRYLAMIDE 000079-06-1 | x | x | | x | | ACRYLONITRILE 000107-13-1 | x | x | x | x | | ACTINOMYCIN D 000050-76-0 ||x| | x | | ADIPONITRILE 000111-69-3 | | | x | | | ADRIAMYCIN 023214-92-8 | x | | | | | AFLATOXIN B1 001162-65-8 | x | | | | | AFLATOXIN M1 006795-23-9 | x | | | | | AFLATOXINS 001402-68-2 | x | | x | | | ALL-TRANS RETINOIC ACID 000302-79-4 | | x | | x | | ALPRAZOMAN 028981-97-7 | | x | | x | | ALUMINUM PHOSPHIDE 020859-73-8 | | | x | | x | AMANTADINE HYDROCHLORIDE 000665-66-7 | | x | | x | | AMINO-2,4-DIBROMOANTHRAQUINONE 000081-49-2 | x | | | | | AMINO-2-METHYLANTHRAQUINONE, 1- 000082-28-0 | x | | | | | AMINO-3,4-DIMETHYL-3h-IMIDAZO(4,5f)QUINOLINE,2- 077094-11-2 | x | | | | | AMINO-3,8-DIMETHYL-3H-IMIDAZO(4,5-f)QUINOXALINE,