Redox Reactions 263
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Structure-Function Relationship of Metalloproteins
RESEARCH ACTIVITIES Structure-Function Relationship of Metalloproteins Department of Life and Coordination-Complex Molecular Science Division of Biomolecular Functions FUJII, Hiroshi Associate Professor KURAHASHI, Takuya Assistant Professor CONG, Zhiqi IMS Fellow OHTSUKI, Akimichi Post-Doctoral Fellow WANG, Chunlan Graduate Student TANIZAWA, Misako Secretary Metalloproteins are a class of biologically important A d4 high-spin manganese(III) ion forms a Robin-Day Class II macromolecules, which have various functions such as oxygen mixed-valence system, in which electron transfer is occurring transport, electron transfer, oxidation, and oxygenation. These between the localized phenoxyl radical and the phenolate. This diverse functions of metalloproteins have been thought to is in clear contrast to a d8 low-spin nickel(II) ion with the depend on the ligands from amino acid, coordination struc- same salen ligand, which induces a delocalized radical (Robin- tures, and protein structures in immediate vicinity of metal Day Class III) over the two phenolate rings, as previously ions. In this project, we are studying the relationship between reported by others. The present findings point to a fascinating the electronic structures of the metal active sites and reactivity possibility that electron transfer could be drastically modu- of metalloproteins. lated by exchanging the metal ion that bridges the two redox centers. 1. One-Electron Oxidation of One-electron Oxidation Electronically-Diverse Manganese(III) and N N N N Nickel(II) Salen Complexes: Transition from M M R O O R R O O R Localized to Delocalized Mixed-Valence tBu tBu 3+ 2+ tBu tBu Ligand Radicals1) M = Mn , Ni t R = Bu, OCH3, Ph Electron Transfer Ligand radicals from salen complexes are unique mixed- Figure 1. -

Redox Models in Chemistry
Redox models in chemistry A depiction of the conceptions held by secondary school students of redox reactions Lise-Lotte Österlund Department of Chemistry 901 87 Umeå Umeå 2010 Copyright©Lise-Lotte Österlund ISBN: 978-91-7459-053-1 ISSN: 1652-5051 Cover picture: Photographer, Lise-Lotte Österlund Printed by: VMC, KBC, Umeå University Umeå, Sweden 2010 T0 my family Abstract According to previous research, students show difficulties in learning redox reactions. By the historical development different redox models exist to explain redox reactions, the oxygen model, the hydrogen model, the electron model and the oxidation number model. This thesis reports about three studies concerning conceptions held by secondary school students of redox reactions. A textbook analysis is also included in the thesis. The first study was an investigation of the students’ use of redox models in inorganic contexts, their use of the activity series of metals, and the students’ ability to transfer redox knowledge. Then the students’ work with an open- ended biochemical task, where the students had access of the textbook was studied. The students talk about redox reactions, the questions raised by the students, what resources used to answer the questions and what kind of talk developed were investigated. A textbook analysis based on chemistry books from Sweden and one book from England was performed. The redox models used as well as the dealing with redox related learning difficulties was studied. Finally, the students’ conceptions about redox in inorganic, organic and biochemistry after completed chemistry courses were studied. The results show that the students were able to use the electron model as a tool to explain inorganic redox reactions and the mutuality of oxidation and reduction was fundamental. -

Boron-Nitrogen Compounds
springer.com Chemistry : Inorganic Chemistry Niedenzu, Kurt, Dawson, J.W. Boron-Nitrogen Compounds Although the chemistry of boron is still relatively young, it is developing at a pace where even specific areas of research are difficult to compile into a monograph. Besides the boron hydrides, boron-nitrogen compounds are among the most fascinating derivatives of boron. Nitrogen compounds exist in a wide variety of molecular structures and display many interesting properties. The combination of nitrogen and boron, however, has some unusual features that are hard to match in any other combination of elements. This situation was first recognized by ALFRED STOCK and it seems proper to pay tribute to his outstanding work in the area of boron chemistry. One should realize that about forty years ago, STOCK and his coworkers had to develop completely new experimental techniq'\les and that no guidance for the interpreta• tion of their rather unusual data had been advanced by theoretical chemists. In this monograph an attempt has been made to explore the general characteristics of structure and the principles involved in the preparation and reactions of boron-nitroge~ compounds. It was a somewhat difficult task to select that information which appears to be of the most Springer interest to "inorganic and general chemistry" since the electronic relationship between a boron- nitrogen and a carbon-carbon grouping is reflected in the "organic" character of many of the Softcover reprint of the 1st reactions and compounds. original 1st ed. 1965, VIII, -

Promotion Effects and Mechanism of Alkali Metals and Alkaline Earth
Subscriber access provided by RES CENTER OF ECO ENVIR SCI Article Promotion Effects and Mechanism of Alkali Metals and Alkaline Earth Metals on Cobalt#Cerium Composite Oxide Catalysts for N2O Decomposition Li Xue, Hong He, Chang Liu, Changbin Zhang, and Bo Zhang Environ. Sci. Technol., 2009, 43 (3), 890-895 • DOI: 10.1021/es801867y • Publication Date (Web): 05 January 2009 Downloaded from http://pubs.acs.org on January 31, 2009 More About This Article Additional resources and features associated with this article are available within the HTML version: • Supporting Information • Access to high resolution figures • Links to articles and content related to this article • Copyright permission to reproduce figures and/or text from this article Environmental Science & Technology is published by the American Chemical Society. 1155 Sixteenth Street N.W., Washington, DC 20036 Environ. Sci. Technol. 2009, 43, 890–895 Promotion Effects and Mechanism such as Fe-ZSM-5 are more active in the selective catalytic reduction (SCR) of N2O by hydrocarbons than in the - ° of Alkali Metals and Alkaline Earth decomposition of N2O in a temperature range of 300 400 C (3). In recent years, it has been found that various mixed Metals on Cobalt-Cerium oxide catalysts, such as calcined hydrotalcite and spinel oxide, showed relatively high activities. Composite Oxide Catalysts for N2O One of the most active oxide catalysts is a mixed oxide containing cobalt spinel. Calcined hydrotalcites containing Decomposition cobalt, such as Co-Al-HT (9-12) and Co-Rh-Al-HT (9, 11), have been reported to be very efficient for the decomposition 2+ LI XUE, HONG HE,* CHANG LIU, of N2O. -

Status and Prospects of Organic Redox Flow Batteries Toward Sustainable Energy Storage Jian Luo,† Bo Hu,† Maowei Hu,† Yu Zhao,‡ and T
Review Cite This: ACS Energy Lett. 2019, 4, 2220−2240 http://pubs.acs.org/journal/aelccp Status and Prospects of Organic Redox Flow Batteries toward Sustainable Energy Storage Jian Luo,† Bo Hu,† Maowei Hu,† Yu Zhao,‡ and T. Leo Liu*,† † The Department of Chemistry and Biochemistry, Utah State University, Logan, Utah 84322, United States ‡ Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China ABSTRACT: Redox flow batteries (RFBs) are regarded a promising technology for large-scale electricity energy storage to realize efficient utilization of intermittent renewable energy. Redox -active materials are the most important components in the RFB system because their physicochemical and electrochemical proper- ties directly determine their battery performance and energy storage cost. Designable, tunable, and potentially low-cost redox- active organic compounds are promising alternatives to traditional redox-active inorganic compounds for RFB applications. Herein, the representative designs of redox-active molecules, recent development of organic RFBs (ORFBs), and advantages/ disadvantages of different ORFB are reviewed. Especially the relationship between redox-active molecules’ physicochemical properties and their battery performance is discussed with an emphasis on the side reactions that cause fading of battery capacity. Finally, we provide an outlook on the development of high-performance ORFBs for practical energy storage -

Process for Purification of Ethylene Compound Having Fluorine-Containing Organic Group
Office europeen des brevets (fi) Publication number : 0 506 374 A1 @ EUROPEAN PATENT APPLICATION @ Application number: 92302586.0 @ Int. CI.5: C07C 17/38, C07C 21/18 (g) Date of filing : 25.03.92 (30) Priority : 26.03.91 JP 86090/91 (72) Inventor : Kishita, Hirofumi 3-19-1, Isobe, Annaka-shi Gunma-ken (JP) (43) Date of publication of application : Inventor : Sato, Shinichi 30.09.92 Bulletin 92/40 3-5-5, Isobe, Annaka-shi Gunma-ken (JP) Inventor : Fujii, Hideki (84) Designated Contracting States : 3-12-37, Isobe, Annaka-shi DE FR GB Gunma-ken (JP) Inventor : Matsuda, Takashi 791 ~4 YdndSG Anri3k3~shi @ Applicant : SHIN-ETSU CHEMICAL CO., LTD. Gunma-ken (JP) 6-1, Ohtemachi 2-chome Chiyoda-ku Tokyo (JP) @) Representative : Votier, Sidney David et al CARPMAELS & RANSFORD 43, Bloomsbury Square London WC1A 2RA (GB) (54) Process for purification of ethylene compound having fluorine-containing organic group. (57) A process for purifying an ethylene compound having a fluorine-containing organic group (fluorine- containing ethylene compound) by mixing the fluorine-containing ethylene compound with an alkali metal or alkaline earth metal reducing agent, and subjecting the resulting mixture to irradiation with ultraviolet radiation, followed by washing with water. The purification process ensures effective removal of iodides which are sources of molecular iodine, from the fluorine-containing ethylene compound. < h- CO CO o 10 o Q_ LU Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 506 374 A1 BACKGROUND OF THE INVENTION 1. Field of the Invention 5 The present invention relates to a process for purifying an ethylene compound having a fluorine-containing organic group, and more particularly to a purification process by which iodine contained as impurity in an ethylene compound having a fluorine-containing organic group can be removed effectively. -
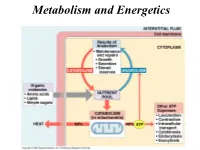
Metabolism and Energetics Oxidation of Carbon Atoms of Glucose Is the Major Source of Energy in Aerobic Metabolism
Metabolism and Energetics Oxidation of carbon atoms of glucose is the major source of energy in aerobic metabolism C6H1206 + 6O2 yields 6 CO2 + H20 + energy Energy released ΔG = - 686 kcal/mol Glucose oxidation requires over 25 discrete steps, with production of 36 ATP. Energy Transformations The mitochondrial synthesis of ATP is not stochiometric. Electron–motive force Proton-motive force Phosphoryl-transfer potential in the form of ATP. Substrate level phosphorylation The formation of ATP by substrate-level phosphorylation ADP ATP P CH O P CH2 O P 2 is used to represent HC OH HC OH a phosphate ester: phosphoglycerate CH OH OH CH2 O P kinase 2 P O bisphosphoglycerate 3-phosphoglycerate OH ADP ATP CH2 CH2 CH3 C O P C OH C O pyruvate kinase non-enzymic COOH COOH COOH phosphoenolpyruvate enolpyruvate pyruvate Why ATP? The reaction of ATP hydrolysis is very favorable ΔGo = - 30.5 kJ/mol = - 7.3 kCal/mol 1. Charge separation of closely packed phosphate groups provides electrostatic relief. Mg2+ 2. Inorganic Pi, the product of the reaction, is immediately resonance-stabilized (electron density spreads equally to all oxygens). 3. ADP immediately ionizes giving H+ into a low [H+] environment (pH~7). 4. Both Pi and ADP are more favorably solvated by water than one ATP molecule. 5. ATP is water soluble. The total body content of ATP and ADP is under 350 mmol – about 10 g, BUT … the amount of ATP synthesized and used each day is about 150 mol – about 110 kg. ATP Production - stage 1 - Glycolysis Glycolysis When rapid production of ATP is needed. -

Molecular Modeling of the Reduction Mechanism in the Citrate- Mediated Synthesis of Gold Nanoparticles Isaac Ojea-Jimeneź † and Josep M
Article pubs.acs.org/JPCC Molecular Modeling of the Reduction Mechanism in the Citrate- Mediated Synthesis of Gold Nanoparticles Isaac Ojea-Jimeneź † and Josep M. Campanera‡,* † Centre d’Investigacióen Nanociencià i Nanotecnologia (CIN2, ICN-CSIC), Campus UAB, 08193, Cerdanyola del Valles,̀ Spain ‡ Departament de Fisicoquímica, Facultat de Farmacia,̀ Universitat de Barcelona, Av. Joan XXIII, s/n, Diagonal Sud, 08028 Barcelona, Spain *S Supporting Information ABSTRACT: The synthesis of gold nanoparticles (Au NPs) via the reduction of tetrachloroauric acid by sodium citrate has become a standard procedure in nanotechnology. Simultaneously, gold- mediated reactions are gaining interest due to their catalytic properties, unseen in other metals. In this study, we have investigated the theoretical mechanism of this reaction under three different pH conditions (acid, mild acid, and neutral) and have corroborated our findings with experimental kinetic measurements by UV−vis absorption spectroscopy and transmission electron microscopy (TEM) analysis of the final particle morphology. We have demonstrated that, indeed, the pH of the medium ultimately determines the reaction rate of the reduction, which is the rate-limiting step in the Au NPs formation and involves decarboxylation of the citrate. The pH sets the dominant species of each of the reactants and, consequently, the reaction pathways slightly differ in each pH condition. The mechanism highlights the effect of the number of Cl− ligands in the metallocomplex, which ultimately originates the energetic -

Curriculum Vitae Professor Dr. Martin Jansen
Curriculum Vitae Professor Dr. Martin Jansen Name: Martin Jansen Born: 5 November 1944 Main areas of research: preparative solid-state chemistry, crystal chemistry, materials research, structure-property relationship of solids Since 1998, he has been a member of the scientific council of the Max Planck Society and a director at the Max Planck Institute for solid-state research in StuttgartHe has developed a concept for plan- ning solid state syntheses, combining computational and experimental tools, that is pointing the way to rational and efficient discovery of new materials. Academic and Professional Career since 1998 Director at the Max Planck Institute for Solid State Research, Stuttgart and Honorary Professor at the University of Stuttgart, Germany 1987 - 1998 Professor (C4) and Director of the Institute at the University of Bonn, Germany 1981 - 1987 Professor (C4), Chair B for Inorganic Chemistry of the University of Hannover, Germany 1978 Habilitation at the University of Gießen, Germany 1973 Promotion (Ph.D.) at the University of Gießen, Germany 1966 - 1970 Study of Chemistry at the University of Gießen, Germany Honours and Awarded Memberships (Selection) 2019 Otto-Hahn-Prize 2009 Centenary Prize, Royal Society of Chemistry, UK 2009 Georg Wittig - Victor Grignard Prize, Société Chimique de France 2008 Member of acatech (National Academy of Science and Engineering) Nationale Akademie der Wissenschaften Leopoldina www.leopoldina.org 1 2007 Karl Ziegler Award, Germany 2004 Honorary Doctorate of the Ludwig Maximilians-University of -

AP Chemistry Textbook : Chemistry: a Molecular Approach 4Th Ed. Nivaldo Tro. Course : Chemistry 130 Is a Study of Fundamental Ch
AP Chemistry Textbook : Chemistry: A Molecular Approach 4th ed. Nivaldo Tro. Course: Chemistry 130 is a study of fundamental chemistry principles, including atomic structure, chemical bonding, kinetic theory, chemical kinetics, thermodynamics, solutions, electrochemistry, nuclear chemistry and equilibrium. Recommended for pre-professional, engineering and related science and medicine majors. I hope to create a similar college classroom experience to prepare the students for the next steps they will take in college. Students will learn through teacher lead discussions as well as laboratory inquiry experiences throughout the year. The course is structured around the enduring understandings within the six big ideas described in the AP Chemistry Curriculum Framework. Attendance : The students are expected to be a class on time ready to learn everyday. As a part of this course there will be a one day per week zero hour lab section starting at 7:00 am. Students are expected to be on time and if there is a prelab exercise it is due upon arrival. If it is not completed the student will not be able to participate in the laboratory activity. Grading: The grades will be weighted in this class. ● Homework/Quizzes 15% ● Exams (~ 3 exams/9 weeks) 55% ● Labs 30% (25% of the instructional time will be devoted to laboratory experience) Students will be performing labs and lab reports in line with expectations of an introductory college level chemistry course and allow students to apply the seven science practices of the AP Framework. They will focus on developing good laboratory technique as well as an inquiry and guided inquiry approach to performing in lab. -

(1) Title: Gas-Phase Oxidation and Disproportionation of Nitric Oxide
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Community Repository of Fukui 1 Title (1) Title: Gas-phase oxidation and disproportionation of nitric oxide (2) Running title: Gas-phase oxidation and disproportionation of NO (3) Authors: Hirokazu Tsukahara*, Takanobu Ishida†, Mitsufumi Mayumi* *Department of Pediatrics, Faculty of Medicine, Fukui Medical University, Fukui 910-1193, Japan †Department of Chemistry, State University of New York at Stony Brook, New York 11794-3400, USA (4) Correspondence: Hirokazu Tsukahara, M.D., Ph.D. Department of Pediatrics, Faculty of Medicine, Fukui Medical University, Fukui 910-1193, Japan Telephone: 81-776-61-3111 (ex. 2316), FAX: 81-776-61-8129 E-mail: [email protected] 2 Introduction Nitrogen and oxygen together comprise over 98% of the air we breathe. Nitric oxide (NO) is a simple molecule, consisting of a single nitrogen bonded to one oxygen atom, which makes its chemistry accessible to study in great detail.1,2 However, it is only recently that mammalian cells were discovered to produce NO as a short-lived intercellular messenger.3,4 NO participates in blood pressure control, neurotransmission and inflammation. Moreover, NO is the biologically active species released from a variety of cardiovascular drugs such as nitroglycerin and isosorbide dinitrate.5 One important function of NO is in the macrophage-dependent killing of invaders, and possibly cancer cells, indicating the potential of this free radical to mediate cytotoxic and pathological effects.6 When inhaled, NO acts as a selective pulmonary vasodilator. There is intense clinical interest in inhalation of low doses of NO (less than 1 to 80 ppm) in the treatment of diseases characterized by pulmonary hypertension and hypoxemia (Table I).7,8 The inhaled NO therapy is fairly inexpensive, but it seems that it is not indicated for everybody with regards to the paradigm of its efficiency and potential toxicity. -

04. Si Monsters
IV The Chemistry of Bug-Eyed Silicon Monsters 1. The Rise and Fall of an Analogy Carbon and silicon were not always regarded as isova- lent analogs of one another. The great Swedish chem- ist, Jöns Jakob Berzelius (figure 1), who was the first to isolate silicon as a simple substance in 1823, thought that it most resembled boron (1, 2). This assignment was based on the fact that both elements formed acidic, nonvolatile oxides which could act as glass formers, and on a similarity in the appearance of the simple substances themselves, both of which had been pre- pared only as highly-impure, amorphous, nonmetallic powders. This analogy was further reinforced by errors in the determination of their atomic weights, which assigned the analogous formulas, BO3 and SiO3, to their respective oxides, in sharp contrast to the formu- las, CO and CO2, assigned to the oxides of carbon. With the gradual correction of atomic weights and the equally gradual substitution of “stoichiometric type” or valence, in place of acidity and electronegativity, as the preferred basis for chemical classification, silicon was reassigned as an analog of carbon. In 1857, the German chemist, Friedrich Wöhler (figure 2), discovered silicon tetrahydride (SiH4), the stoichiometric and structural analog of methane (CH4), and the logical starting point for speculations on an Figure 2. Friedrich Wöhler (1800-1882). alternative organic chemistry based on silicon rather than carbon (3). Ironically, however, Wöhler did not consider this possibility until 1863 and then only as a result of a faulty interpretation of his experimental data. Having obtained, via the hydrolysis of magne- sium silicide, a series of apparent compounds of sili- con, hydrogen and oxygen, he found it very difficult to assign them exact formulas.