Participants PP (Including the Commission) Restricted to a Group Defined by the Consortium RE (Including the Commission)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ergosterol Purified from Medicinal Mushroom Amauroderma Rude Inhibits Cancer Growth in Vitro and in Vivo by Up-Regulating Multiple Tumor Suppressors
www.impactjournals.com/oncotarget/ Oncotarget, Vol. 6, No. 19 Ergosterol purified from medicinal mushroom Amauroderma rude inhibits cancer growth in vitro and in vivo by up-regulating multiple tumor suppressors Xiangmin Li1,2,3,4,*, Qingping Wu2,*, Yizhen Xie2, Yinrun Ding2, William W. Du3,4, Mouna Sdiri3,4, Burton B. Yang3,4 1School of Bioscience and Bioengineering, South China University of Technology, Guangzhou 510006, PR China 2State Key Laboratory of Applied Microbiology Southern China (The Ministry-Province Joint Development), Guangdong Institute of Microbiology, Guangzhou, 510070, PR China 3Sunnybrook Research Institute, Sunnybrook Health Sciences Centre, Toronto, M4N3M5, Canada 4Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, M4N3M5, Canada *These authors have contributed equally to this work Correspondence to: Yizhen Xie, e-mail: [email protected] Burton B. Yang, e-mail: [email protected] Keywords: herbal medicine, medicinal mushroom, Foxo3a, Bim, Fas Received: April 08, 2015 Accepted: May 13, 2015 Published: May 27, 2015 ABSTRACT We have previously screened thirteen medicinal mushrooms for their potential anti-cancer activities in eleven different cell lines and found that the extract of Amauroderma rude exerted the highest capacity in inducing cancer cell death. The current study aimed to purify molecules mediating the anti-cancer cell activity. The extract of Amauroderma rude was subject to fractionation, silica gel chromatography, and HPLC. We purified a compound and identified it as ergosterol by EI-MS and NMR, which was expressed at the highest level in Amauroderma rude compared with other medicinal mushrooms tested. We found that ergosterol induced cancer cell death, which was time and concentration dependent. -
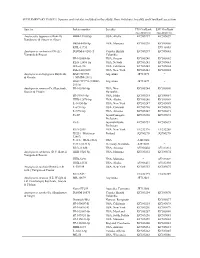
Suppl Table I
SUPPLEMENTARY TABLE I. Species and isolates included in the study, their vouchers, locality and GenBank accession Species Isolate number Locality ITS GenBank LSU GenBank accession no. accession no. Amylocystis lapponica (Romell) HHB-13400-Sp USA, Alaska KC585237 KC585059 Bondartsev & Singer ex Singer OKM-4418-Sp USA, Montana KC585238 KC585060 KHL-11755 − EU118603 Amyloporia carbonica (Overh.) DAOM-F-8281-T Canada, British KC585239 KC585061 Vampola & Pouzar Columbia FP-105585-Sp USA, Oregon KC585240 KC585062 RLG-12496-Sp USA, Nevada KC585241 KC585063 Wilcox-96 USA, California KC585242 KC585064 Zabel-40-GLN USA, New York KC585243 KC585065 Amyloporia nothofaginea Rajchenb. BAFC519794 Argentina JF713078 − & Gorjón (=MMBP-2011) BAFC519796 (=MBP- Argentina JF713079 − 2011a) Amyloporia sinuosa (Fr.) Rajchenb., FP-105386-Sp USA, New KC585244 KC585066 Gorjón & Pildain Hampshire FP-94464-Sp USA, Idaho KC585245 KC585067 HHB-12878-Sp USA, Alaska KC585246 KC585068 L-14130-Sp USA, New York KC585247 KC585069 L-6192-Sp USA, Colorado KC585248 KC585070 L-9792-Sp USA, Arizona KC585249 KC585071 Pa-3C JapanYamagata KC585250 KC585072 Prefecture Pa-3e JapanShizuoka KC585251 KC585073 Prefecture RLG-2538 USA, New York EU232196 EU232288 X725 (=Miettinen- Finland JQ700270 JQ700270 12407) P-115 (=RLG-2538) USA AJ416068 − P-211 (G-214) Germany, Karlsrube AJ345011 − RLG-1182R USA, Arizona AY966450 AY333831 Amyloporia sitchensis (D.V. Baxter) HHB-5320-Sp USA, Montana KC585252 KC585074 Vampola & Pouzar HHB-5298 USA, Montana − AY333829 HHB-12513 USA, Alaska AY966451 AY333830 -

Why Mushrooms Have Evolved to Be So Promiscuous: Insights from Evolutionary and Ecological Patterns
fungal biology reviews 29 (2015) 167e178 journal homepage: www.elsevier.com/locate/fbr Review Why mushrooms have evolved to be so promiscuous: Insights from evolutionary and ecological patterns Timothy Y. JAMES* Department of Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, MI 48109, USA article info abstract Article history: Agaricomycetes, the mushrooms, are considered to have a promiscuous mating system, Received 27 May 2015 because most populations have a large number of mating types. This diversity of mating Received in revised form types ensures a high outcrossing efficiency, the probability of encountering a compatible 17 October 2015 mate when mating at random, because nearly every homokaryotic genotype is compatible Accepted 23 October 2015 with every other. Here I summarize the data from mating type surveys and genetic analysis of mating type loci and ask what evolutionary and ecological factors have promoted pro- Keywords: miscuity. Outcrossing efficiency is equally high in both bipolar and tetrapolar species Genomic conflict with a median value of 0.967 in Agaricomycetes. The sessile nature of the homokaryotic Homeodomain mycelium coupled with frequent long distance dispersal could account for selection favor- Outbreeding potential ing a high outcrossing efficiency as opportunities for choosing mates may be minimal. Pheromone receptor Consistent with a role of mating type in mediating cytoplasmic-nuclear genomic conflict, Agaricomycetes have evolved away from a haploid yeast phase towards hyphal fusions that display reciprocal nuclear migration after mating rather than cytoplasmic fusion. Importantly, the evolution of this mating behavior is precisely timed with the onset of diversification of mating type alleles at the pheromone/receptor mating type loci that are known to control reciprocal nuclear migration during mating. -

A New Pericarbonyl Lignan from Amauroderma Rude
ORIGINAL ARTICLE Rec. Nat. Prod. 13:4 (2019) 296-300 A New Pericarbonyl Lignan from Amauroderma rude Miao Dong 1, Zuhong Ma 2, Qiaofen Yang 2, Qiuyue Hu 2, Yanqing Ye 2,* and Min Zhou 1,* 1Key Laboratory of Chemistry in Ethnic Medicinal Resources, State Ethnic Affairs Commission & Ministry of Education, Yunnan Minzu University, Kunming 650031, P.R. China 2 School of Chemistry and Environment, Yunnan Minzu University, Kunming 650031, P.R. China (Received October 24, 2018; Revised November 29, 2018; Accepted November 30, 2018) Abstract: A new pericarbonyl lignan (1), named amaurolignan A was isolated from an ethanol extract of the fruiting bodies in Amauroderma rude of family Ganodermataceae, together with two known lignans, 4-methoxymatairesinol 4′-β-D-glucoside (2) and lappaol F (3). The structures of compounds (1-3) were elucidated using NMR and MS spectroscopic methods. Keywords: Pericarbonyl lignan; amaurolignan A; Amauroderma rude. © 2019 ACG Publications. All rights reserved. 1. Introduction “Lingzhi” is a mushroom that has been renowned in China for more than 2000 years because of its claimed medicinal properties and symbolic fortune, which translates as ‘Ganodermataceae’ in a broad sense, and in a narrow sense it represents the highly prized medicinal Ganoderma species distributed in East Asia [1]. Its medicinal properties include anti-aging, lowering blood pressure, improving immunity, and preventing and treating various cancers, chronic bronchitis, gastric ulcers, hepatitis, neurasthenia and thrombosis [2-4]. The medicinal effects of many mushrooms such as Ganoderma lucidum, Lentinula edodes, Agaricus blazei, Antrodia camphorate and Grifola frondosaI come from their metabolites including polysaccharides, triterpenes, lucidenic acids, adenosine, ergosterol, glucosamine and cerebrosides [5-8]. -

Oxalic Acid Degradation by a Novel Fungal Oxalate Oxidase from Abortiporus Biennis Marcin Grąz1*, Kamila Rachwał2, Radosław Zan2 and Anna Jarosz-Wilkołazka1
Vol. 63, No 3/2016 595–600 http://dx.doi.org/10.18388/abp.2016_1282 Regular paper Oxalic acid degradation by a novel fungal oxalate oxidase from Abortiporus biennis Marcin Grąz1*, Kamila Rachwał2, Radosław Zan2 and Anna Jarosz-Wilkołazka1 1Department of Biochemistry, Maria Curie-Skłodowska University, Lublin, Poland; 2Department of Genetics and Microbiology, Maria Curie-Skłodowska University, Lublin, Poland Oxalate oxidase was identified in mycelial extracts of a to formic acid and carbon dioxide (Mäkelä et al., 2002). basidiomycete Abortiporus biennis strain. Intracellular The degradation of oxalate via action of oxalate oxidase enzyme activity was detected only after prior lowering (EC 1.2.3.4), described in our study, is atypical for fun- of the pH value of the fungal cultures by using oxalic or gi and was found predominantly in higher plants. The hydrochloric acids. This enzyme was purified using size best characterised oxalate oxidase originates from cereal exclusion chromatography (Sephadex G-25) and ion-ex- plants (Dunwell, 2000). Currently, only three oxalate oxi- change chromatography (DEAE-Sepharose). This enzyme dases of basidiomycete fungi have been described - an exhibited optimum activity at pH 2 when incubated at enzyme from Tilletia contraversa (Vaisey et al., 1961), the 40°C, and the optimum temperature was established at best characterised so far enzyme from Ceriporiopsis subver- 60°C. Among the tested organic acids, this enzyme ex- mispora (Aguilar et al., 1999), and an enzyme produced by hibited specificity only towards oxalic acid. Molecular Abortiporus biennis (Grąz et al., 2009). The enzyme from mass was calculated as 58 kDa. The values of Km for oxa- C. -

Phylogenetic Classification of Trametes
TAXON 60 (6) • December 2011: 1567–1583 Justo & Hibbett • Phylogenetic classification of Trametes SYSTEMATICS AND PHYLOGENY Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five-marker dataset Alfredo Justo & David S. Hibbett Clark University, Biology Department, 950 Main St., Worcester, Massachusetts 01610, U.S.A. Author for correspondence: Alfredo Justo, [email protected] Abstract: The phylogeny of Trametes and related genera was studied using molecular data from ribosomal markers (nLSU, ITS) and protein-coding genes (RPB1, RPB2, TEF1-alpha) and consequences for the taxonomy and nomenclature of this group were considered. Separate datasets with rDNA data only, single datasets for each of the protein-coding genes, and a combined five-marker dataset were analyzed. Molecular analyses recover a strongly supported trametoid clade that includes most of Trametes species (including the type T. suaveolens, the T. versicolor group, and mainly tropical species such as T. maxima and T. cubensis) together with species of Lenzites and Pycnoporus and Coriolopsis polyzona. Our data confirm the positions of Trametes cervina (= Trametopsis cervina) in the phlebioid clade and of Trametes trogii (= Coriolopsis trogii) outside the trametoid clade, closely related to Coriolopsis gallica. The genus Coriolopsis, as currently defined, is polyphyletic, with the type species as part of the trametoid clade and at least two additional lineages occurring in the core polyporoid clade. In view of these results the use of a single generic name (Trametes) for the trametoid clade is considered to be the best taxonomic and nomenclatural option as the morphological concept of Trametes would remain almost unchanged, few new nomenclatural combinations would be necessary, and the classification of additional species (i.e., not yet described and/or sampled for mo- lecular data) in Trametes based on morphological characters alone will still be possible. -

Short-Read Sequencing for Genomic Analysis of the Brown Rot Fungus Fibroporia Radiculosa
Short-Read Sequencing for Genomic Analysis of the Brown Rot Fungus Fibroporia radiculosa Juliet D. Tang,a Andy D. Perkins,b Tad S. Sonstegard,c Steven G. Schroeder,c Shane C. Burgess,d and Susan V. Diehla Forest Products, Mississippi State University, Mississippi State, Mississippi, USAa; Computer Science and Engineering, Mississippi State University, Mississippi State, Mississippi, USAb; USDA ARS Bovine Functional Genomics Laboratory, Beltsville, Maryland, USAc; and Institute for Genomics, Biocomputing, and Biotechnology, Mississippi State University, Mississippi State, Mississippi, USAd The feasibility of short-read sequencing for genomic analysis was demonstrated for Fibroporia radiculosa, a copper-tolerant fungus that causes brown rot decay of wood. The effect of read quality on genomic assembly was assessed by filtering Illumina GAIIx reads from a single run of a paired-end library (75-nucleotide read length and 300-bp fragment size) at three different stringency levels and then assembling each data set with Velvet. A simple approach was devised to determine which filter strin- gency was “best.” Venn diagrams identified the regions containing reads that were used in an assembly but were of a low-enough quality to be removed by a filter. By plotting base quality histograms of reads in this region, we judged whether a filter was too stringent or not stringent enough. Our best assembly had a genome size of 33.6 Mb, an N50 of 65.8 kb for a k-mer of 51, and a maximum contig length of 347 kb. Using GeneMark, 9,262 genes were predicted. TargetP and SignalP analyses showed that among the 1,213 genes with secreted products, 986 had motifs for signal peptides and 227 had motifs for signal anchors. -

A New Species of Antrodia (Basidiomycota, Polypores) from China
Mycosphere 8(7): 878–885 (2017) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/8/7/4 Copyright © Guizhou Academy of Agricultural Sciences A new species of Antrodia (Basidiomycota, Polypores) from China Chen YY, Wu F* Institute of Microbiology, Beijing Forestry University, Beijing 100083, China Chen YY, Wu F 2017 –A new species of Antrodia (Basidiomycota, Polypores) from China. Mycosphere 8(7), 878–885, Doi 10.5943/mycosphere/8/7/4 Abstract A new species, Antrodia monomitica sp. nov., is described and illustrated from China based on morphological characters and molecular evidence. It is characterized by producing annual, fragile and nodulose basidiomata, a monomitic hyphal system with clamp connections on generative hyphae, hyaline, thin-walled and fusiform to mango-shaped basidiospores (6–7.5 × 2.3– 3 µm), and causing a typical brown rot. In phylogenetic analysis inferred from ITS and nLSU rDNA sequences, the new species forms a distinct lineage in the Antrodia s. l., and has a close relationship with A. oleracea. Key words – Fomitopsidaceae – phylogenetic analysis – taxonomy – wood-decaying fungi Introduction Antrodia P. Karst., typified with Polyporus serpens Fr. (=Antrodia albida (Fr.) Donk (Donk 1960, Ryvarden 1991), is characterized by a resupinate to effused-reflexed growth habit, white or pale colour of the context, a dimitic hyphal system with clamp connections on generative hyphae, hyaline, thin-walled, cylindrical to very narrow ellipsoid basidiospores which are negative in Melzer’s reagent and Cotton Blue, and causing a brown rot (Ryvarden & Melo 2014). Antrodia is a highly heterogeneous genus which is closely related to Fomitopsis P. -

Three Species of Wood-Decaying Fungi in <I>Polyporales</I> New to China
MYCOTAXON ISSN (print) 0093-4666 (online) 2154-8889 Mycotaxon, Ltd. ©2017 January–March 2017—Volume 132, pp. 29–42 http://dx.doi.org/10.5248/132.29 Three species of wood-decaying fungi in Polyporales new to China Chang-lin Zhaoa, Shi-liang Liua, Guang-juan Ren, Xiao-hong Ji & Shuanghui He* Institute of Microbiology, Beijing Forestry University, No. 35 Qinghuadong Road, Haidian District, Beijing 100083, P.R. China * Correspondence to: [email protected] Abstract—Three wood-decaying fungi, Ceriporiopsis lagerheimii, Sebipora aquosa, and Tyromyces xuchilensis, are newly recorded in China. The identifications were based on morphological and molecular evidence. The phylogenetic tree inferred from ITS+nLSU sequences of 49 species of Polyporales nests C. lagerheimii within the phlebioid clade, S. aquosa within the gelatoporia clade, and T. xuchilensis within the residual polyporoid clade. The three species are described and illustrated based on Chinese material. Key words—Basidiomycota, polypore, taxonomy, white rot fungus Introduction Wood-decaying fungi play a key role in recycling nutrients of forest ecosystems by decomposing cellulose, hemicellulose, and lignin of the plant cell walls (Floudas et al. 2015). Polyporales, a large order in Basidiomycota, includes many important genera of wood-decaying fungi. Recent molecular studies employing multi-gene datasets have helped to provide a phylogenetic overview of Polyporales, in which thirty-four valid families are now recognized (Binder et al. 2013). The diversity of wood-decaying fungi is very high in China because of the large landscape ranging from boreal to tropical zones. More than 1200 species of wood-decaying fungi have been found in China (Dai 2011, 2012), and some a Chang-lin Zhao and Shi-liang Liu contributed equally to this work and share first-author status 30 .. -

Basidiomycota) in Finland
Mycosphere 7 (3): 333–357(2016) www.mycosphere.org ISSN 2077 7019 Article Doi 10.5943/mycosphere/7/3/7 Copyright © Guizhou Academy of Agricultural Sciences Extensions of known geographic distribution of aphyllophoroid fungi (Basidiomycota) in Finland Kunttu P1, Kulju M2, Kekki T3, Pennanen J4, Savola K5, Helo T6 and Kotiranta H7 1University of Eastern Finland, School of Forest Sciences, P.O. Box 111, FI-80101 Joensuu, Finland 2Biodiversity Unit P.O. Box 3000, FI-90014 University of Oulu, Finland 3Jyväskylä University Museum, Natural History Section, P.O. BOX 35, FI-40014 University of Jyväskylä, Finland 4Pentbyntie 1 A 2, FI-10300 Karjaa, Finland 5The Finnish Association for Nature Conservation, Itälahdenkatu 22 b A, FI-00210 Helsinki, Finland 6Erätie 13 C 19, FI-87200 Kajaani, Finland 7Finnish Environment Institute, P.O. Box 140, FI-00251 Helsinki, Finland Kunttu P, Kulju M, Kekki T, Pennanen J, Savola K, Helo T, Kotiranta H 2016 – Extensions of known geographic distribution of aphyllophoroid fungi (Basidiomycota) in Finland. Mycosphere 7(3), 333–357, Doi 10.5943/mycosphere/7/3/7 Abstract This article contributes the knowledge of Finnish aphyllophoroid funga with nationally or regionally new species, and records of rare species. Ceriporia bresadolae, Clavaria tenuipes and Renatobasidium notabile are presented as new aphyllophoroid species to Finland. Ceriporia bresadolae and R. notabile are globally rare species. The records of Ceriporia aurantiocarnescens, Crustomyces subabruptus, Sistotrema autumnale, Trechispora elongata, and Trechispora silvae- ryae are the second in Finland. New records (or localities) are provided for 33 species with no more than 10 records in Finland. In addition, 76 records of aphyllophoroid species are reported as new to some subzones of the boreal vegetation zone in Finland. -

Phylum Order Number of Species Number of Orders Family Genus Species Japanese Name Properties Phytopathogenicity Date Pref
Phylum Order Number of species Number of orders family genus species Japanese name properties phytopathogenicity date Pref. points R inhibition H inhibition R SD H SD Basidiomycota Polyporales 98 12 Meruliaceae Abortiporus Abortiporus biennis ニクウチワタケ saprobic "+" 2004-07-18 Kumamoto Haru, Kikuchi 40.4 -1.6 7.6 3.2 Basidiomycota Agaricales 171 1 Meruliaceae Abortiporus Abortiporus biennis ニクウチワタケ saprobic "+" 2004-07-16 Hokkaido Shari, Shari 74 39.3 2.8 4.3 Basidiomycota Agaricales 269 1 Agaricaceae Agaricus Agaricus arvensis シロオオハラタケ saprobic "-" 2000-09-25 Gunma Kawaba, Tone 87 49.1 2.4 2.3 Basidiomycota Polyporales 181 12 Agaricaceae Agaricus Agaricus bisporus ツクリタケ saprobic "-" 2004-04-16 Gunma Horosawa, Kiryu 36.2 -23 3.6 1.4 Basidiomycota Hymenochaetales 129 8 Agaricaceae Agaricus Agaricus moelleri ナカグロモリノカサ saprobic "-" 2003-07-15 Gunma Hirai, Kiryu 64.4 44.4 9.6 4.4 Basidiomycota Polyporales 105 12 Agaricaceae Agaricus Agaricus moelleri ナカグロモリノカサ saprobic "-" 2003-06-26 Nagano Minamiminowa, Kamiina 70.1 3.7 2.5 5.3 Basidiomycota Auriculariales 37 2 Agaricaceae Agaricus Agaricus subrutilescens ザラエノハラタケ saprobic "-" 2001-08-20 Fukushima Showa 67.9 37.8 0.6 0.6 Basidiomycota Boletales 251 3 Agaricaceae Agaricus Agaricus subrutilescens ザラエノハラタケ saprobic "-" 2000-09-25 Yamanashi Hakusyu, Hokuto 80.7 48.3 3.7 7.4 Basidiomycota Agaricales 9 1 Agaricaceae Agaricus Agaricus subrutilescens ザラエノハラタケ saprobic "-" 85.9 68.1 1.9 3.1 Basidiomycota Hymenochaetales 129 8 Strophariaceae Agrocybe Agrocybe cylindracea ヤナギマツタケ saprobic "-" 2003-08-23 -

Biodiversity of Wood-Decay Fungi in Italy
AperTO - Archivio Istituzionale Open Access dell'Università di Torino Biodiversity of wood-decay fungi in Italy This is the author's manuscript Original Citation: Availability: This version is available http://hdl.handle.net/2318/88396 since 2016-10-06T16:54:39Z Published version: DOI:10.1080/11263504.2011.633114 Terms of use: Open Access Anyone can freely access the full text of works made available as "Open Access". Works made available under a Creative Commons license can be used according to the terms and conditions of said license. Use of all other works requires consent of the right holder (author or publisher) if not exempted from copyright protection by the applicable law. (Article begins on next page) 28 September 2021 This is the author's final version of the contribution published as: A. Saitta; A. Bernicchia; S.P. Gorjón; E. Altobelli; V.M. Granito; C. Losi; D. Lunghini; O. Maggi; G. Medardi; F. Padovan; L. Pecoraro; A. Vizzini; A.M. Persiani. Biodiversity of wood-decay fungi in Italy. PLANT BIOSYSTEMS. 145(4) pp: 958-968. DOI: 10.1080/11263504.2011.633114 The publisher's version is available at: http://www.tandfonline.com/doi/abs/10.1080/11263504.2011.633114 When citing, please refer to the published version. Link to this full text: http://hdl.handle.net/2318/88396 This full text was downloaded from iris - AperTO: https://iris.unito.it/ iris - AperTO University of Turin’s Institutional Research Information System and Open Access Institutional Repository Biodiversity of wood-decay fungi in Italy A. Saitta , A. Bernicchia , S. P. Gorjón , E.