Goddard Space Flight Center Battery Workshop
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fortnight Nears the End
World Bridge Series Championship Philadelphia Pennsylvania, USA 1st to 16th October D B 2010 aily ulletin O FFICIAL S PONSOR Co-ordinator: Jean-Paul Meyer • Chief Editor: Brent Manley • Editors: Mark Horton, Brian Senior, Phillip Alder, Barry Rigal, Jan Van Cleef • Lay Out Editor: Akis Kanaris • Photographer: Ron Tacchi Issue No. 14 Friday, 15 October 2010 FORTNIGHT NEARS THE END These are the hard-working staff members who produce all the deals — literally thousands — for the championships Players at the World Bridge Series Championships have been In the World Junior Championship, Israel and France will start at it for nearly two weeks with only one full day left. Those play today for the Ortiz-Patino Trophy, and in the World Young- who have played every day deserve credit for their stamina. sters Championship, it will be England versus Poland for the Consider the players who started on opening day of the Damiani Cup. Generali Open Pairs on Saturday nearly a week ago. If they made it to the final, which started yesterday, they will end up playing 15 sessions. Contents With three sessions to go, the Open leaders, drop-ins from the Rosenblum, are Fulvio Fantoni and Claudio Nunes. In the World Bridge Series Results . .3-5 Women’s Pairs, another pair of drop-ins, Carla Arnolds and For Those Who Like Action . .6 Bep Vriend are in front. The IMP Pairs leaders are Joao-Paulo Campos and Miguel Vil- Sting in the Tail . .10 las-Boas. ACBL President Rich DeMartino and Patrick McDe- Interview with José Damiani . .18 vitt are in the lead in the Hiron Trophy Senior Pairs. -

Gateway to the West Regional Sunday
Sunday July 14-19 Hi 92°F Low 75°F Daily Bulletin Gateway to the West Regional All St. Louis Regional Results: for coming to St. Louis and we’d like www.acbl.org & www.unit143.org, to see you right back here again next Unit 143 includes links to the week’s Daily Bulletins. year. We appreciate that you chose to attend our Regional ’coz we do it all for you! to our Caddies, We appreciate your fine work this week! Jackson Florea Anna Garcia Jenna Percich Lauren Percich Clara Riggio Frank Riggio Katie Seibert Kate Vontz Our Date Back to August 15-21, 2016 Come back and join us next August. Please put us on your Regional tournament calendar today. Charity Pairs Series Raises $ BackStoppers will receive the $$$$ that you helped us raise in the Saturday morning Charity Open Pairs Game and will be added to what Last Chance for Registration Gift & was raised in the Wednesday evening Swiss event. We support this To Pick Up Your Section Top Awards organization to express our appreciation for lives given on behalf of Sunday, from 10:00 – 10:20 AM before the Swiss Team session others. Unit 143 will present the check at their October Sectional. begins, and 30 minutes after the sessions end, will be the last opportunity to pick up your convention card holder and section Thanks for playing in these events and showing your support! top awards. Daily Grin How can you tell if someone is a lousy bridge player? No Peeking, Lew! He has 5 smiling Kibitzers watching him play. -

The Minor ALT INVITATIONAL IV & Tampalt Qualification
Minor ALT IV BULLETIN 4 • Friday November 20 • editor Christina Lund Madsen • [email protected] The minor ALT INVITATIONAL IV & TampAlt Qualification NOVEMBER 16-20 2020 WORLD CLASS ONLINE BRIDGE EVENTS The Mugs made it Yesterday we said goodbye to 28 teams. In the Minor Alt the four remaining teams are Fredin vs. Red Devils and Moss vs. Gupta. Honourable mention goes to Black, defeated by the Red Devils, De Michelis who succombed to Fredin, Eastwest who ran into the Moss wall and Wilson, who need 11 more IMPs against Gupta. The Mugs (Jon Cooke, Kay Preddy, Norman Selway, Cameron Small, Jeremy Willans) and Vinita (Dennis Bilde, Soren Bilde, Alon Birman, Vinita Gupta, Hemant Lall) hung on to their positions as first and second respectively on the final day of the TampAlt Qualification. Both teams win a free entry to the TampAlt main event Photo: Peter Hasenson December 14-18. Congratulations! To the left is Kay Preddy, one of the Mugs. Important Notice Today’s Schedule Minor Alt All players should enter BBO 10 Friday November 20 minutes before their match starts at 10:00 EST / 16:00 CET – Semifinal (28 boards) the latest. Tournament director Denis Dobrin is waiting for you and will 14:30 EST / 20:30 CET – Final (32 boards) instruct you where to sit. - 1 - Results Minor Alt Invitational IV Round Robin Quarterfinals Semifinals All Results - 2 - Final Result TampAlt Qualification Next ALT event The TampAlt main event takes place December 14-18. This is a Major Alt event for up to 32 teams and still open to new entries. -

Fantoni-Nunes Notes by Daniel Neill Source
Fantoni-Nunes notes by Daniel Neill source: - Vugraph Project (http://www.sarantakos.com/bridge/vugraph.html) - BBO myhands last updated: 9-20-07 news: 1M-2C-2D/S rearrangement Opening Summary: 1C = 15+ balanced (5D-332 ok), or 14+ value 5+C/4441, F1 1D = 14+ value 5+D or 444-1C/1S, F1 1H = 14+ value 5+H (12+ if 4S), F1 1S = 14+ value 5+S (12+ if 4+H), F1 1N = 12-14, any 5422 ok except both M's, 6m ok, all 4441's (!) 2C = 10-13 value, 5C-4other unbalanced, or 6+C (5C-5S has opened 2C before) 2D = 10-13 value, 5D-4M/4+m unbalanced, or 6+D 2M = 10-13 value, 5M-4+m unbalanced, or 6+M 2N = 21-22 bal 3y/4y = pree 3N = was solid 7+crd minor nothing on side Upgrade often. 2/1 GF (2C 3-way), unless if opener has 5-4+ majors. Third/Fourth Seat - 2-bids are (6)8-12 (mb 5332), 1-bids 13+, 2/1 not GF X/XX by unlimited hand shows cards while bids all show minimums, even jumps. Responses: ******************** Opening 1C Responses ******************** 1D = 4+H, 0-11 (X) XX = 18+, cards (takeout doubles) 1H = 4+H 14-17/GF, or 23+ NT - forcing (X) 2m = as normal 1S = was 3433/2533/2443/1525 max 2C = was 4423 20 3C = was 1525 (etc etc) 2H = was 15 4H bal twice 4H = to play 2C = short D: 5+ if sing. or 7+ if doub. 2D = short C: 5+ if sing. -

49Th Asia Pacific Bridge Federation Championships
49th Asia Pacific Bridge Federation Championships Bulletin 9 Bulletin Editor: Mark Horton, Layout Editor: Christine Law 15 June 2013 Photographer: Pin Yuen Wai Champions! Japan at the Double and Australia Don’t forget to duck! In the Open Teams Japan, Indonesia, China and Chinese Taipei cruised into the top four places. With Japan as In this issue Champions and Indonesia already qualified as hosts Thailand Scheduled Matches 2 and Singapore also go into the playoffs. In the Ladies series Japan and Indonesia finished well clear. Today’s programme 5 By defeating Chinese Taipei and New Zealand Australia Open Teams Thailand v Chinese Taipei 6 secured third place. China held on to fourth by defeating the Servant of Two Masters 9 Chinese Taipei, who finished fifth, still good enough to earn Every Hand an Adventure 12 them a playoff spot along with China Hong Kong and Seating Arrangement 17 Philippines. Australia-Australia just pipped Indonesia to top the Results of Open Teams 20 Senior's table. Then came Chinese Taipei. Results of Ladies Teams 21 China HK - Tse, China-China Evertrust , Japan Imax Results of Senior Teams 22 and Thailand 1 will contest the playoffs. Ranking 23 Full details of the playoff schedule are in today's Bulletin. Acknowledgement 24 Meanwhile, in the Zone 7 play offs Australia meet New Zealand in all three contests. Today’s VuGraph Matches 10:00 China v Chinese Taipei (Open) 13:30 To be decided 16:00 To be decided 18:30 To be decided Regal Hongkong Hotel , Causeway Bay, Hong Kong 7 - 16 June 2013 49th Asia Pacific Bridge -

Young Turks Win Pairs
1st1st WorldWorld MindMind SportsSports GamesGames YouthYouth TeamTeam ChampionshipsChampionships Beijing-ChinaBeijing-China 33rd-18-18th OctoberOctober 20082008 Chief Editor: Brian SENIOR Editors: Micke MELANDER - Jan van CLEEFF Layout Editor: Panagiotis PAPADOPOULOS Photographer: Wattanai CHANAKOT Bulletin 12 Thursday, 16 October 2008 YOUNGYOUNG TURKSTURKS WINWIN PAIRSPAIRS he new World Junior Pairs champions are Melih Osman Sen and Mehmet Remzi Sakirler from Vugraph Turkey. The last two boards proved to be decisive T There will be a vugraph since the young Turks were almost equal with Ron presentation from today’s Schwarz and Lotan Fisher from Israel. On the penulti- Individual Championship, mate board the Israelis went down in slam while the morning session only. Turkish pair made 3NT with three overtricks. The last board, however, looked splendid for Ron and Lotan, as they made 6NT with an overtrick, while with good def- Today’s Schedule ence only twelve tricks were available. Israel high fived, 10.30 Individual, Session 1 but the party all of a sudden stopped, when rumours spread that Sen and Remzi bid and made 7NT on the 15.30 Individual, Session 2 board. And so it proved to be. After winning the Euro- pean Senior Championships this year in Pau, this meant a second huge success for Turkish bridge. 1st WORLD MIND SPORTS GAMES Beijing, China MIRZA ASFANDIYAR HUSSAIN The Youth event has come and gone but one remark- able player of only 15 years came, played and went back What’s your strength and weakness without being noticed exc- as a bridge player? ept by those who played against him. -

District 4Spot
2011 DISTRICT 4 WEBSITE WWW.DISTRICT4.INFO Unit Websites 112 - acblunit112.org 120 - nepab.com 121 - unit121bridge.com 133-lvbabridge.org 141 - philadelphiabridge.info 168 - http://web2.acbl.org/hosted/units/unit168/index.htm 190 - unit190.org .217 - unit217.nepab.com PRESIDENT RICK ROWLAND [email protected] 1ST VICE PRESIDENT WALTER MITCHELL 2ND VICE PRESIDENT TOM WEIK SECRETARY EDITOR BILL BAUER Elaine Landow TREASURER 2556 Morris Road RICK ROWLAND Lansdale, PA 19446 DISTRICT DIRECTOR 215-699-6134 CRAIG ROBINSON e-mail: 215-699-6134 [email protected] [email protected] All rights reserved. No part of this publication may be reproduced without permission of the editor. 2 District 4Spot PRESIDENT’S MESSAGE RICK ROWLAND The Syracuse regional gave me the opportunity to meet many new folks from the northern part of the district. Gerry Radway, Margie Spence and their committee put together a wonderful event. The Valley Forge regional was smaller than we had hoped for, but has a great new venue. The Dolce Valley Forge and its staff were very accommodating. As Carole King once said, I felt the earth move under my feet. Tuesday’s earthquake was a bit scary for those of us who had never experienced one before, but the bridge went on. Continuing with the 70’s music theme, Gloria Gaynor wrote “I will survive”. Unfortunately, the tournament didn’t survive Hurricane Irene. Out of an abundance of caution, the District 4 Board voted to cancel Saturday night and Sunday bridge sessions based on information available at the time. I look forward to seeing you all in Lancaster. -

Worldyouth Bridgeteams Championships
WORLDYOUTH 15TH BRIDGETEAMS CHAMPIONSHIPS KoçUniversityIstanbulTurkey13th23rdAugust2014 DAILY BULLETIN Editor: Phillip Alder • Co-Editors: Micke Melander, David Stern • Lay-out Editor: Francesca Canali Bulletin No. 9 Friday, 22 August, 2014 IT’S NOW OR NEVER: THE THREE FINALS JUNIORS: YOUNGSTERS: GIRLS NETHERLANDS - NORWAY USA1 - SWEDEN FRANCE - CHINA he finals are beginning this morning, but only after In the match between France and the Netherlands, the T some tense semifinals yesterday afternoon. Dutch led after each session, although a small matter of 77 imps were exchanged in the last set. The winning In the Girls division, China was down by 0.5 imps at margin was 21 imps. halftime against the Netherlands. But China won both of the remaining sessions to come home by 13.5 imps. The finals are over 84 boards today and tomorrow. The In the France-Italy match, France led by 39 imps with third-place playoffs are only 56 boards long. one 14-board session to be played. Then Italy had a stea- dy stream of gains that fell just short, by 5 imps. Or had The Board-a-Match Teams also ends today. they? The Italians registered an appeal on a board, but after lengthy deliberation, the result stood and France were through. Departure Details Sweden had an easy win by 75.5 imps over Norway in the Youngsters event. USA1 triumphed over France by Before leaving, you must check out in Building S. 20.5 imps, although there were some anxious moments for their supporters near the end. And when the departure sheets go up today, please check them carefully to see that you are listed correctly. -

W Om En's Team “Trials” and Tribulations
Volume 1, Issue 3 May 25, 2009 Mancuso vs Dinkin in the Finals SemiFinals 25-MAY-2009 # TEAM TOTAL 1-16 17-32 33-48 49-64 USBF President Bill Pollack 1 Dinkin 124 39 33 21 31 USBF 4 Glasson 97 23 13 12 49 Vice President Steve Beatty 2 Mancuso 123 9 33 39 42 USBF Secretary 3 Moss 107 32 23 27 25 Joan Gerard USBF Treasurer Sylvia Moss Peaches Picks: One and One… USBF Chief Operations Officer Glasson lost by 27 … A Poor result for me… Jan Martel But it was Plane to see that Peggy would Pull through on an American USBF Chief holiday. Financial Officer Barbara Nudelman Mancuso won by 16 … and it’s Dinkin and Mancuso in the finals. Director - USWBC Terry Lavender I wasn’t Perfect, but there time to imProve … As I said, Mancuso’s flying high and I’m picking them to go all the way. No need for seat cushion Round Robin flotation devices on this ride … it will be smooth sailing as they Play for Director a berth in Sao Paulo. Pam Hughes In the Playoff 1, it’s Glasson and Moss. I still Prefer Glasson and I’m Appeals Coordinator sticking to my guns. Look for Glasson and Dinkin in the race for USA2. Joan Gerard And Please, Pour me a Pink Lady and let me get some sleeP. These late VuGraph nights and VuGraph Perils are exhausting. Coordinator Jan Martel Till tomorrow… Bulletin Editor SleeP tight Payton… Remember the Power of P … Suzi Subeck Hospitality Chairs If you hold ♠AJ654 ♥T ♦QT32 ♣KT9 and it goes: Pass, Pass, four hearts, Nadine Wood Pass, Pass, SAY PASS! Lynn Johannesen It will be more Perfect than going –790 if Partner won’t Pick a suit!! Webmaster Kitty Cooper UNITED STATES and Tribulations “Trials” Team Women’s WOMEN’S BRIDGE CHAMPIONSHIPS 1 WOMEN’S TEAM “TRIALS” AND TRIBULATIONS A Minor Difference.. -

Senior Gold for Canada/USA
Co-ordinator: Jean-Paul Meyer (France) Issue: 11 Chief Editor: Mark Horton (England) Editors: Brent Manley (USA), Brian Senior (England) Layout Editor: George Hatzidakis (Greece) Photographer: Ron Tacchi (England) 27th August 2002 Senior Gold for Canada/USA George Mittelman, Boris Baran, Diana Holt, Ed Schulte (pictured above) & Joe Godefrin are the new World Se- World Bridge Federation - nior Champions, winning an exciting final against Ameri- Master Point Certificates ca's Chris Larsen, Joe Kivel, Gene Freed, Nels Erikson, Players in the Can-Am Regional Tournament are getting two Lew Finkel & Bernie Miller. for the price of one, being awarded both ACBL and WBF Masterpoints here at the World Championships. The bronze medal went to The Netherlands Roald For the events completed to date, the WBF Masterpoint Ramer, Jan Willem Bomhof and Henk & Elly Schippers. Certificates are now available for collection from the Regis- Today's McConnell final is an all USA affair, Radin v San- tration Desk in the Queen Elizabeth Hotel.They are attrac- tively presented on good quality laminated paper and well- born. In the Power Rosenblum we are down to four worth having as a souvenir of the Championships. teams, from Indonesia, Poland, Sweden and Italy. VuGraph Salle François ground floor ATTENTION!!! 10.00 Burgay - Munawar No food or drink allowed in the Vugraph. 12.40 Lavazza - Fredin 15.50 to be decided Aucune nourriture ni boisson permise 18.30 to be decided dans la salle de ramma. 2 2002 World Bridge Championships POWER ROSENBLUM CUP (Quarter-Finals) -
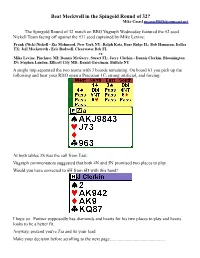
Beat Meckwell in the Spingold Round of 32? Mike Cassel [email protected]
Beat Meckwell in the Spingold Round of 32? Mike Cassel [email protected] The Spingold Round of 32 match on BBO Vugraph Wednesday featured the #2 seed Nickell Team facing off against the #31 seed captained by Mike Levine: Frank (Nick) Nickell - Zia Mahmood, New York NY; Ralph Katz, Burr Ridge IL; Bob Hamman, Dallas TX; Jeff Meckstroth - Eric Rodwell, Clearwater Bch FL vs Mike Levine, Pinckney MI; Dennis McGarry, Stuart FL; Jerry Clerkin - Dennis Clerkin, Bloomington IN; Stephen Landen, Ellicott City MD; Daniel Gerstman, Buffalo NY A single imp separated the two teams with 3 boards remaining. On board 61 you pick up the following and hear your RHO open a Precision 1C, strong artificial, and forcing: At both tables 3S was the call from East. Vugraph commentators suggested that both 4N and 5N promised two places to play. Would you have corrected to 6H from 6D with this hand? I hope so. Partner supposedly has diamonds and hearts for his two places to play and hearts looks to be a better fit. Anyway, pretend you're Zia and its your lead. Make your decision before scrolling to the next page................................................. The heroic?, get your name in the daily bulletin, lead is the S9 right? Partner has raised spades and if he holds the Q you are going to get a diamond ruff for the best possible result. Here was the auction at the other table There are a lot of imps available because your counterparts jammed the auction leading to a different contract. Your lead is? Underleading your SA is a huge error, lose 14, if you let your spade trick escape. -

Bulletin 4.Qxd
Co-ordinator: Jean Paul Meyer – Editor: Mark Horton – Assistant Editors: Brent Manley & Brian Senior French Editor: Guy Dupont – Layout Editor: Stelios Hatzidakis – Photographer: Ron Tacchi Issue No. 4 PDF version, courtesy of EBL Thursday, 25 October 2001 Half Way Round the Robin VUGRAPH MATCHES Bermuda Bowl – ROUND 10 – 10.00 Indonesia v Poland Bermuda Bowl – ROUND 11 – 14.00 Norway v USA I Bermuda Bowl – ROUND 12 – 17.20 France v Italy Contents Marc Hodler follows the action on VuGraph Bermuda Bowl Program & Results . 2 Venice Cup Program & Results . 2-3 As we move into the second half of the Round Robin Bermuda Bowl & Venice Cup Ranking . 3 the Bermuda Bowl race is headed by USA II and Poland. Seniors Bowl Program . 3 In the Venice Cup France are setting the pace from Eng- Bermuda Bowl (Poland v Norway) . 4 land. In the Seniors Bowl France are now the only unbeat- Echos du Stade . 9 en team in all three competitions but they are only just Venice Cup (Israel v Australia) . 10 Duck Or No Dinner . 12 ahead of Italy. Seniors Bowl Results, Ranking & Butler . 13 Championship Diary . 13 Distinguished Visitors Bermuda Bowl (USA I v Argentina) . 14 It is with great pleasure that we extend a very warm wel- Bermuda Bowl & Venice Cup Butler . 16 come to two distinguished guests to these 2001 World Bridge Championships who are honouring us with their presence. Jaime Ortiz-Patiño, President Emeritus of the WBF, needs Press Conference no introduction. There will be a Press Conference today at 11.00 in the Marc Hodler, Vice President of the International Olympic Chorum - access via entrance E - given by Marc Hodler, Committee and a member of the new IOC Executive Com- IOC Vice President, Jimmy Ortiz Patiño, President Emeri- mission, has been a very good friend to the WBF.