Scorpine-Like Peptides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Small Molecules in the Venom of the Scorpion Hormurus Waigiensis
biomedicines Article Small Molecules in the Venom of the Scorpion Hormurus waigiensis Edward R. J. Evans 1, Lachlan McIntyre 2, Tobin D. Northfield 3, Norelle L. Daly 1 and David T. Wilson 1,* 1 Centre for Molecular Therapeutics, AITHM, James Cook University, Cairns, QLD 4878, Australia; [email protected] (E.R.J.E.); [email protected] (N.L.D.) 2 Independent Researcher, P.O. Box 78, Bamaga, QLD 4876, Australia; [email protected] 3 Department of Entomology, Tree Fruit Research and Extension Center, Washington State University, Wenatchee, WA 98801, USA; [email protected] * Correspondence: [email protected]; Tel.: +61-7-4232-1707 Received: 30 June 2020; Accepted: 28 July 2020; Published: 31 July 2020 Abstract: Despite scorpion stings posing a significant public health issue in particular regions of the world, certain aspects of scorpion venom chemistry remain poorly described. Although there has been extensive research into the identity and activity of scorpion venom peptides, non-peptide small molecules present in the venom have received comparatively little attention. Small molecules can have important functions within venoms; for example, in some spider species the main toxic components of the venom are acylpolyamines. Other molecules can have auxiliary effects that facilitate envenomation, such as purines with hypotensive properties utilised by snakes. In this study, we investigated some non-peptide small molecule constituents of Hormurus waigiensis venom using LC/MS, reversed-phase HPLC, and NMR spectroscopy. We identified adenosine, adenosine monophosphate (AMP), and citric acid within the venom, with low quantities of the amino acids glutamic acid and aspartic acid also being present. -

The Predation of an Adult Colubrid Snake, Sibynophis Triangularis, by the Scorpion Heterometrus Laoticus in the Sakaerat Biosphere Reserve
Captive & Field Herpetology Volume 4 Issue 1 2020 Inverting the Food Web: The Predation of an Adult Colubrid Snake, Sibynophis triangularis, by the Scorpion Heterometrus laoticus in the Sakaerat Biosphere Reserve Jack Christie1, Everett Madsen1, Surachit Waengsothorn1, Max Dolton Jones1, 2,* 1Sakaerat Environmental Research Station, Nakhon Ratchasima, Thailand. 2Suranree University of Technology, Nakhon Ratchasima, Thailand. *Corresponding author, e-mail: [email protected] Abstract: system they occupy, is a well-researched and understood topic (Belovsky & Slade 1993; The predation of vertebrates by large tropical Murkin & Batt 1987; Nordberg et al. 2018). invertebrates is a rare phenomenon in nature, This particularly pertains to the predation of though not unprecedented. Scorpions in invertebrates by larger vertebrate predators. particular are evolutionarily equipped to take However, there are far fewer instances of on such a task. Despite the hunting strategy invertebrates consuming vertebrate prey items and primarily insectivorous diet of scorpions, within the available literature. Scorpions are they are known to opportunistically prey upon primarily fossorial ambush predators, emerging small vertebrate prey such as lizards, frogs and from their burrows and lying in wait at the birds. Herein, we describe the observation of entrance for unsuspecting prey to wander too an adult Asian forest scorpion (Heterometrus close (Williams 1987). Scorpions typically laoticus; Scorpiones; Scorpionidae) predating exhibit an insectivorous diet, with the upon an adult triangle many-toothed snake exception of a few species (Williams 1987), (Sibynophis triangularis; Squamata: and have been known to prey on frogs, birds, Colubridae). Although the opportunistic lizards and even snakes (McCormick & Polis, predation on snakes by scorpions has been 1982). -

Sexual Dimorphism in the Asian Giant Forest Scorpion, Heterometrus Laoticus Couzijn, 1981
NU Science Journal 2007; 4(1): 42 - 52 Sexual Dimorphism in the Asian Giant Forest Scorpion, Heterometrus laoticus Couzijn, 1981 Ubolwan Booncham1*, Duangkhae Sitthicharoenchai2, Art-ong Pradatsundarasar2, Surisak Prasarnpun1 and Kumthorn Thirakhupt2 1Department of Biology, Faculty of Science, Naresuan University, Phitsanulok 65000 Thailand 2Department of Biology, Faculty of Science, Chulalongkorn University, Bangkok 10400 Thailand *Corresponding author. E-mail address: [email protected] ABSTRACT Morphological characters of adult male and adult female giant forest scorpions, Heterometrus laoticus, in a mixed deciduous forest at Phitsanulok Wildlife Conservation Development and Extension Station showed sexual dimorphism. Among the observed characters, carapace width, chela length, chela width, telson length and shape of movable finger of adult male and female scorpions were obviously different. The pectines of males were also significantly longer, and the number of sensilla-bearing teeth in male scorpions was more than in females. Moreover, males had higher density of sensilla on the pectinal teeth than females. During the breeding season, mature males were mobile while mature females were mainly at their burrows. Keywords: Heterometrus laoticus, sexual dimorphism INTRODUCTION Sexual dimorphism is the difference in form between males and females of the same species. Sexual dimorphism, particularly sexual size dimorphism (SSD) has been observed in a large number of animal taxa (Blanckenhorn, 2005; Brown, 1996; David et al., 2003; Esperk and Tammaru, 2006; Herrel et al. 1999; Ozkan et al., 2006; Ranta et al. 1994; Shine, 1989; Walker and Rypstra, 2001 and Wangkulangkul, et al., 2005). Under the influence of natural and sexual selections, males and females often differ in costs and benefits of achieving some particular body sizes (Crowley, 2000; Gaffin and Broenell, 2001; Kladt, 2003; Mattoni, 2005). -

Target-Specificity in Scorpions
Toxins 2017, 9, 312, doi: 10.3390/toxins9100312 S1 of S3 Supplementary Materials: Target‐specificity in scorpions; Comparing lethality of scorpion venoms across arthropods and vertebrates Arie van der Meijden, Bjørn Koch, Tom van der Valk, Leidy J. Vargas‐Muñoz and Sebastian Estrada‐Gómez Table S1. Table with GenBank CO1 accession numbers. Voucher numbers refer to the specimens in the collection of CIBIO/InBio at the University of Porto. Species Voucher Accession number Androctonus australis Sc904 gi379647585 Leiurus quinquestriatus Sc1062 gi379647601 Buthus ibericus Sc112 gi290918312 Grosphus flavopiceus Sc1085 gi379647593 Centruroides gracilis gi71743793 Heterometrus laoticus Sc1084 gi555299473 Pandinus imperator Sc1050 gi379647587 Hadrurus arizonensis Sc1042 gi379647597 Iurus kraepelini Sc866 gi379647589 Table S2. Mean dry venom compound per individual. Several milkings were made per (sub)adult specimen in some cases. Species n Dry venom (mg) mg/milking Androctonus australis 21 23.5 1.12 Leiurus quinquestriatus 51 25.1 0.49 Buthus ibericus 47 41.6 0.89 Centruroides gracilis 42 22.5 0.54 Babycurus jacksoni 38 53.9 1.42 Grosphus grandidieri 19 103.9 5.47 Hadrurus arizonensis 9 75.3 8.37 Iurus sp.* 12 35.2 2.93 Heterometrus laoticus 21 119 5.67 Pandinus imperator 15 72.7 4.85 *2 I. dufoureius, 6 I. kraepelini, 4 I. sp. Toxins 2017, 9, 312, doi: 10.3390/toxins9100312 S2 of S3 Table S3. Toxicological analysis of the venom of Grosphus grandidieri. “Toxic” means that the mice showed symptoms such as: pain, piloerection, excitability, salivation, lacrimation, dyspnea, diarrhea, temporary paralysis, but recovered within 20 h. “Lethal” means that the mice showed some or all the symptoms of intoxication and died within 20 h after injection. -

Caracterização Proteometabolômica Dos Componentes Da Teia Da Aranha Nephila Clavipes Utilizados Na Estratégia De Captura De Presas
UNIVERSIDADE ESTADUAL PAULISTA “JÚLIO DE MESQUITA FILHO” INSTITUTO DE BIOCIÊNCIAS – RIO CLARO PROGRAMA DE PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS BIOLOGIA CELULAR E MOLECULAR Caracterização proteometabolômica dos componentes da teia da aranha Nephila clavipes utilizados na estratégia de captura de presas Franciele Grego Esteves Dissertação apresentada ao Instituto de Biociências do Câmpus de Rio . Claro, Universidade Estadual Paulista, como parte dos requisitos para obtenção do título de Mestre em Biologia Celular e Molecular. Rio Claro São Paulo - Brasil Março/2017 FRANCIELE GREGO ESTEVES CARACTERIZAÇÃO PROTEOMETABOLÔMICA DOS COMPONENTES DA TEIA DA ARANHA Nephila clavipes UTILIZADOS NA ESTRATÉGIA DE CAPTURA DE PRESA Orientador: Prof. Dr. Mario Sergio Palma Co-Orientador: Dr. José Roberto Aparecido dos Santos-Pinto Dissertação apresentada ao Instituto de Biociências da Universidade Estadual Paulista “Júlio de Mesquita Filho” - Campus de Rio Claro-SP, como parte dos requisitos para obtenção do título de Mestre em Biologia Celular e Molecular. Rio Claro 2017 595.44 Esteves, Franciele Grego E79c Caracterização proteometabolômica dos componentes da teia da aranha Nephila clavipes utilizados na estratégia de captura de presas / Franciele Grego Esteves. - Rio Claro, 2017 221 f. : il., figs., gráfs., tabs., fots. Dissertação (mestrado) - Universidade Estadual Paulista, Instituto de Biociências de Rio Claro Orientador: Mario Sergio Palma Coorientador: José Roberto Aparecido dos Santos-Pinto 1. Aracnídeo. 2. Seda de aranha. 3. Glândulas de seda. 4. Toxinas. 5. Abordagem proteômica shotgun. 6. Abordagem metabolômica. I. Título. Ficha Catalográfica elaborada pela STATI - Biblioteca da UNESP Campus de Rio Claro/SP Dedico esse trabalho à minha família e aos meus amigos. Agradecimentos AGRADECIMENTOS Agradeço a Deus primeiramente por me fortalecer no dia a dia, por me capacitar a enfrentar os obstáculos e momentos difíceis da vida. -

The Scorpion Fauna of Mona Island, Puerto Rico (Scorpiones: Buthidae, Scorpionidae)
The Scorpion Fauna of Mona Island, Puerto Rico (Scorpiones: Buthidae, Scorpionidae) Rolando Teruel, Mel J. Rivera & Alejandro J. Sánchez August 2017 – No. 250 Euscorpius Occasional Publications in Scorpiology EDITOR: Victor Fet, Marshall University, ‘[email protected]’ ASSOCIATE EDITOR: Michael E. Soleglad, ‘[email protected]’ Euscorpius is the first research publication completely devoted to scorpions (Arachnida: Scorpiones). Euscorpius takes advantage of the rapidly evolving medium of quick online publication, at the same time maintaining high research standards for the burgeoning field of scorpion science (scorpiology). Euscorpius is an expedient and viable medium for the publication of serious papers in scorpiology, including (but not limited to): systematics, evolution, ecology, biogeography, and general biology of scorpions. Review papers, descriptions of new taxa, faunistic surveys, lists of museum collections, and book reviews are welcome. Derivatio Nominis The name Euscorpius Thorell, 1876 refers to the most common genus of scorpions in the Mediterranean region and southern Europe (family Euscorpiidae). Euscorpius is located at: http://www.science.marshall.edu/fet/Euscorpius (Marshall University, Huntington, West Virginia 25755-2510, USA) ICZN COMPLIANCE OF ELECTRONIC PUBLICATIONS: Electronic (“e-only”) publications are fully compliant with ICZN (International Code of Zoological Nomenclature) (i.e. for the purposes of new names and new nomenclatural acts) when properly archived and registered. All Euscorpius issues starting from No. 156 (2013) are archived in two electronic archives: • Biotaxa, http://biotaxa.org/Euscorpius (ICZN-approved and ZooBank-enabled) • Marshall Digital Scholar, http://mds.marshall.edu/euscorpius/. (This website also archives all Euscorpius issues previously published on CD-ROMs.) Between 2000 and 2013, ICZN did not accept online texts as "published work" (Article 9.8). -

Arachnides 76
Arachnides, 2015, n°76 ARACHNIDES BULLETIN DE TERRARIOPHILIE ET DE RECHERCHES DE L’A.P.C.I. (Association Pour la Connaissance des Invertébrés) 76 2015 0 Arachnides, 2015, n°76 LES PREDATEURS DES SCORPIONS (ARACHNIDA : SCORPIONES) G. DUPRE Dans leur revue sur les prédateurs de scorpions, Polis, Sissom & Mac Cormick (1981) relèvent 150 espèces dont essentiellement des espèces adaptées au comportement nocturne de leur proie (chouettes, rongeurs, carnivores nocturnes) mais également des espèces diurnes (lézards, rongeurs, carnivores....) qui débusquent les scorpions sous les pierres ou dans leurs terriers. Dans une précédente note (Dupré, 2008) nous avions effectué un relevé afin d'actualiser cette étude de 1981. Sept ans après, de nouvelles données sont présentées dans cette synthèse. Voici un nouveau relevé des espèces prédatrices. Nous ne faisons pas mention des scorpions qui feront l'objet d'un futur article traité avec le cannibalisme. Explication des tableaux: La première colonne correspond aux prédateurs, la seconde aux régions concernées et la troisième aux références. Dans la mesure du possible, les noms scientifiques ont été rectifiés en fonction des synonymies ou des nouvelles combinaisons appliquées depuis les dates de publication d'origine. ARTHROPODA ARACHNIDA SOLIFUGAE Solifugae Afrique du Nord Millot & Vachon, 1949; Punzo, 1998; Cloudsley-Thompson, 1977 Eremobates sp. USA Bradley, 1983 ARACHNIDA ARANEAE Acanthoscurria atrox Brésil Lourenço, 1981 Aphonopelma sp. et autres Amérique centrale Mazzotti, 1964 Teraphosidae Phormictopus auratus Cuba Teruel & De Armas, 2012 Brachypelma vagans Mexique Dor et al., 2011 Epicadus heterogaster Brésil Lourenço et al. 2006 Latrodectus sp. USA Baerg, 1961 L. hesperus USA Polis et al., 1981 L. mactans Cuba Teruel, 1996; Teruel & De Armas, 2012 L. -

Reanalysis of the Genus Scorpio Linnaeus 1758 in Sub-Saharan Africa and Description of One New Species from Cameroon
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Entomologische Mitteilungen aus dem Zoologischen Museum Hamburg Jahr/Year: 2011 Band/Volume: 15 Autor(en)/Author(s): Lourenco Wilson R. Artikel/Article: Reanalysis of the genus Scorpio Linnaeus 1758 in sub-Saharan Africa and description of one new species from Cameroon (Scorpiones, Scorpionidae) 99-113 ©Zoologisches Museum Hamburg, www.zobodat.at Entomol. Mitt. zool. Mus. Hamburg15(181): 99-113Hamburg, 15. November 2009 ISSN 0044-5223 Reanalysis of the genus Scorpio Linnaeus 1758 in sub-Saharan Africa and description of one new species from Cameroon (Scorpiones, Scorpionidae) W ilson R. Lourenço (with 32 figures) Abstract For almost a century, Scorpio maurus L., 1758 (Scorpiones, Scorpionidae) has been considered to be no more than a widespread and presumably highly polymorphic species. Past classifications by Birula and Vachon have restricted the status of different populations to subspecific level. In the present paper, and in the light of new evidence, several African populations are now raised to the rank of species. One of these, Scorpio occidentalis Werner, 1936, is redescribed and a neotype proposed to stabilise the taxonomy of the group. A new species is also described from the savannah areas of Cameroon. This is the second to be recorded from regions outside the Sahara desert zone. Keywords: Scorpiones, Scorpionidae, Scorpio, new rank, new species, Africa, Cameroon. Introduction The genus Scorpio was created by Linnaeus in 1758 (in part), and has Scorpio maurus Linnaeus, 1758 as its type species, defined by subsequent designation (Karsch 1879; see also Fet 2000). -

Anticoagulant Activity of Low-Molecular Weight Compounds from Heterometrus Laoticus Scorpion Venom
toxins Article Anticoagulant Activity of Low-Molecular Weight Compounds from Heterometrus laoticus Scorpion Venom Thien Vu Tran 1,2, Anh Ngoc Hoang 1, Trang Thuy Thi Nguyen 3, Trung Van Phung 4, Khoa Cuu Nguyen 1, Alexey V. Osipov 5, Igor A. Ivanov 5, Victor I. Tsetlin 5 and Yuri N. Utkin 5,* ID 1 Institute of Applied Materials Science, Vietnam Academy of Science and Technology, Ho Chi Minh City 700000, Vietnam; [email protected] (T.V.T.); [email protected] (A.N.H.); [email protected] (K.C.N.) 2 Vietnam Academy of Science and Technology, Graduate University of Science and Technology, Ho Chi Minh City 700000, Vietnam 3 Faculty of Pharmacy, Nguyen Tat Thanh University, Ho Chi Minh City 700000, Vietnam; [email protected] 4 Istitute of Chemical Technology, Vietnam Academy of Science and Technology, Ho Chi Minh City 700000, Vietnam; [email protected] 5 Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry, Russian Academy of Sciences, Moscow 117997, Russia; [email protected] (A.V.O.); [email protected] (I.A.I.); [email protected] (V.I.T.) * Correspondence: [email protected] or [email protected]; Tel.: +7-495-336-6522 Academic Editor: Steve Peigneur Received: 9 September 2017; Accepted: 21 October 2017; Published: 26 October 2017 Abstract: Scorpion venoms are complex polypeptide mixtures, the ion channel blockers and antimicrobial peptides being the best studied components. The coagulopathic properties of scorpion venoms are poorly studied and the data about substances exhibiting these properties are very limited. During research on the Heterometrus laoticus scorpion venom, we have isolated low-molecular compounds with anticoagulant activity. -

Full-Text (PDF)
Vol. 6(1), pp. 21-28, January 2014 DOI: 10.5897/JMA2013.0286 ISSN 2141-2308 ©2014 Academic Journals Journal of Microbiology and Antimicrobials http://www.academicjournals.org/JMA Full Length Research Paper Investigation of the antimicrobial and hemolytic activity of venom of some Egyptian scorpion Wesam Salama* and Naglaa Geasa Zoology Department, Faculty of Science, Tanta University, Egypt. Accepted 16 January, 2014 Leuirus quinquestriatus, Androctonus amoreuxi and Androctonus australis are venomous scorpion in Egypt. Their venom is complex mixture of salts, neurotoxins, peptides and proteins which has therapeutic applications, and can rapidly kill a broad range of microbes. Estimations of total proteins of the venom indicated that L. quinquestriatus had the highest value than the others (10.64 ± 0.04). Hemolytic activity of human erythrocytes was detected and showed that all concentrations (20, 8 and 5 mg/ml) of tested scorpion species crude venom have hemolytic activity against human erythrocytes. The present study was conducted to evaluate the antimicrobial activity of the three Egyptian scorpion’s venom against four Gram-positive and negative bacterial strains (Bacillus cereus, Bacillus subtillis, Citrobacter freundi and Klibsella pneumonia) in addition to one fungus species Candida albicans. The results show that L. quinquestriatus venom has a significant antibacterial effect against B. subtillis and C. freundi. In contrast, A. amoreuxi and A. australis venoms do not have a noticeable effect on tested microbes. So, the aim of the present study was to investigate the antimicrobial activity of crude venom against Gram-negative, as well as Gram-positive bacteria, fungi and to evaluate the hemolytic activity of the three investigated species on human erythrocytes. -
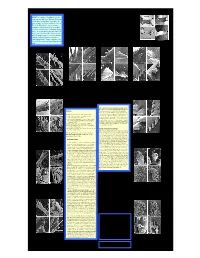
Scorpion Tufts.Pdf
ABSTRACT. Five scorpion genera of superfamily Iuroidea exhibit Tarsal Spinule Clusters and Evolution of the ancient disjunct ranges (South America, North America, Mediterranean), and are an important object in the study of scorpion phylogeny. They have an exceptional variety of tarsal leg setation/spination (Soleglad & Fet 2003). New SEM data from all five genera and two families: Superfamily Iuroidea (Scorpiones) Caraboctonidae (Caraboctonus, Hadruroides, Hadrurus) and Iuridae (Iurus, Calchas) are characterized in detail. We demonstrate two major patterns: (1) an irregular median row of grouped spinule clusters, found in juvenile to subadult but reduced in adult (Calchas); or (2) a median 1 2 3 4 row of highly concentrated spinule clusters. Pattern (2) is either forming Victor Fet , Michael E. Soleglad , David P.A. Neff & Iasmi Stathi “spinule tufts” (Caraboctonus, Hadruroides, Iurus), or individual 1Department of Biological Sciences, and 3Department of Chemistry, Marshall University, Huntington, WV, USA; “spinule-looking” protuberances (Hadrurus). We suggest that the latter Hadrurus obscurus are a derived feature as a result of fusion of separate spinules into a solid 2Borrego Springs, CA, USA; 3Department of Zoology, University of Crete, Irakleio, Crete, Greece structure. General appearance of tarsal armament in Iuroidea Figs. 17-20. Lateral-ventral view of leg III tarsus of Hadrurus a. arizonensis. 17. Figs. 21-24. Lateral-ventral view of leg III (leg IV in Fig. 24) tarsus of Mexican Figs. 25-28. Lateral-ventral view of leg III tarsus of Baja California Hadrurus species. Closeup of fused spinule cluster of adult (carapace length = xx.x mm). Borrego Hadrurus species. 21. Closeup of fused spinule cluster of adult H. -

Soleglad, Fet & Lowe: Hadrurus “Spadix” Subgroup 17
Soleglad, Fet & Lowe: Hadrurus “spadix” Subgroup 17 Figures 31–33 Comparisons of Hadrurus obscurus and H. spadix, metasomal segments II–III, ventral view, showing diagnostic setation located between the ventromedian (VM) carinae. 31. H. obscurus, male (pale phenotype, segment II length = 8.44 mm, segment III length = 9.26 mm), Bird Spring Canyon Road, Kern Co., California, USA. 32. H. obscurus, female (dark phenotype, segment II length = 5.02 mm, segment III length = 5.70 mm), Bird Spring Canyon Road, Kern Co., California, USA. 33. H. spadix, female (segment II length = 7.87 mm, segment III length = 8.36 mm), Apex Mine in Curly Hollow Wash, Washington Co., Utah, USA. 19). However, to support our suspicion, we have ex- As stated above the positions and numbers of chelal amined two H. obscurus specimens collected from the internal trichobothria are essentially identical in Ha- same locality where one has a carapace pattern of H. drurus obscurus and H. spadix, while both numbers and spadix and the other a pattern typical of H. obscurus (see positions differ in H. anzaborrego (see Fig. 19). H. Fig. 20 for color closeup images of these carapaces, and anzaborrego has three internal accessory trichobothria Figs. 9–10 for overall comparison with “arizonensis” whereas the other two species have two. Statistics group species). Based on these data, we suggest here that involving over 250 samples show that these numerical these carapacial pattern differences are analogous to differences are observed in over 87 % of the specimens those exhibited by the dark and pale phenotypes of H. examined (Fig.