Appendix C Dr. Kiviat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2014 Hydrilla Integrated Management
Reviewed January 2017 Publishing Information The University of Florida Institute of Food and Agricultural Sciences (UF/IFAS) is an Equal Opportunity Institution. UF/IFAS is committed to diversity of people, thought and opinion, to inclusiveness and to equal opportunity. The use of trade names in this publication is solely for the purpose of providing specific information. UF/IFAS does not guarantee or warranty the products named, and references to them in this publication do not signify our approval to the exclusion of other products of suitable composition. All chemicals should be used in accordance with directions on the manufacturer’s label. Use pesticides and herbicides safely. Read and follow directions on the manufacturer’s label. For questions about using pesticides, please contact your local county Extension office. Visit http://solutionsforyourlife.ufl.edu/map to find an office near you. Copyright 2014, The University of Florida Editors Jennifer L. Gillett-Kaufman (UF/IFAS) Verena-Ulrike Lietze (UF/IFAS) Emma N.I. Weeks (UF/IFAS) Contributing Authors Julie Baniszewski (UF/IFAS) Ted D. Center (USDA/ARS, retired) Byron R. Coon (Argosy University) James P. Cuda (UF/IFAS) Amy L. Giannotti (City of Winter Park) Judy L. Gillmore (UF/IFAS) Michael J. Grodowitz (U.S. Army Engineer Research and Development Center) Dale H. Habeck, deceased (UF/IFAS) Nathan E. Harms (U.S. Army Engineer Research and Development Center) Jeffrey E. Hill (UF/IFAS) Verena-Ulrike Lietze (UF/IFAS) Jennifer Russell (UF/IFAS) Emma N.I. Weeks (UF/IFAS) Marissa L. Williams (City of Maitland) External Reviewers Nancy L. Dunn (Florida LAKEWATCH volunteer) Stephen D. -
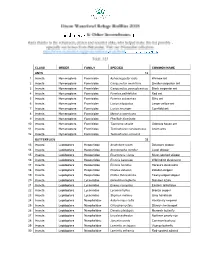
Class Order Family Species Common Name Ants 12 1
CLASS ORDER FAMILY SPECIES COMMON NAME ANTS 12 1 Insecta Hymenoptera Formicidae Aphaenogaster rudis Winnow ant 2 Insecta Hymenoptera Formicidae Camponotus nearcticus Smaller carpenter ant 3 Insecta Hymenoptera Formicidae Camponotus pennsylvanicus Black carpenter ant 4 Insecta Hymenoptera Formicidae Formica pallidefulva Red ant 5 Insecta Hymenoptera Formicidae Formica subsericea Silky ant 6 Insecta Hymenoptera Formicidae Lasius interjectus Larger yellow ant 7 Insecta Hymenoptera Formicidae Lasius neoniger Cornfield ant 8 Insecta Hymenoptera Formicidae Myrmica americana 9 Insecta Hymenoptera Formicidae Pheidole bicarinata 10 Insecta Hymenoptera Formicidae Tapinoma sessile Odorous house ant 11 Insecta Hymenoptera Formicidae Temnothorax curvispinosus Acorn ants 12 Insecta Hymenoptera Formicidae Temnothorax schaumii BUTTERFLIES 32 13 Insecta Lepidoptera Hesperiidae Anatrytone logan Delaware skipper 14 Insecta Lepidoptera Hesperiidae Ancyloxypha numitor Least skipper 15 Insecta Lepidoptera Hesperiidae Epargyreus clarus Silver-spotted skipper 16 Insecta Lepidoptera Hesperiidae Erynnis baptisiae Wild indigo duskywing 17 Insecta Lepidoptera Hesperiidae Erynnis horatius Horace's duskywing 18 Insecta Lepidoptera Hesperiidae Poanes zabulon Zabulon skipper 19 Insecta Lepidoptera Hesperiidae Polites themistocles Tawny-edged skipper 20 Insecta Lepidoptera Lycaenidae Celastrina neglecta Summer azure 21 Insecta Lepidoptera Lycaenidae Everes comyntas Eastern tailed blue 22 Insecta Lepidoptera Lycaenidae Lycaena hyllus Bronze copper 23 Insecta Lepidoptera -

Download the NOAA Fact Nonindigenous Aquatic Nuisance Prevention and Sheet from the Public Domain
Reviewed March 2020 Publishing Information The University of Florida Institute of Food and Agricultural Sciences (UF/IFAS) is an Equal Opportunity Institution. UF/IFAS is committed to diversity of people, thought and opinion, to inclusiveness and to equal opportunity. The use of trade names in this publication is solely for the purpose of providing specific information. UF/IFAS does not guarantee or warranty the products named, and references to them in this publication do not signify our approval to the exclusion of other products of suitable composition. All chemicals should be used in accordance with directions on the manufacturer’s label. Use pesticides and herbicides safely. Read and follow directions on the manufacturer’s label. For questions about using pesticides, please contact your local county Extension office. Visit http://solutionsforyourlife.ufl.edu/map to find an office near you. Copyright 2014, The University of Florida Editors Jennifer L. Gillett-Kaufman (UF/IFAS) Verena-Ulrike Lietze (UF/IFAS) Emma N.I. Weeks (UF/IFAS) Contributing Authors Julie Baniszewski (UF/IFAS) Ted D. Center (USDA/ARS, retired) Byron R. Coon (Argosy University) James P. Cuda (UF/IFAS) Amy L. Giannotti (City of Winter Park) Judy L. Gillmore (UF/IFAS) Michael J. Grodowitz (U.S. Army Engineer Research and Development Center) Dale H. Habeck, deceased (UF/IFAS) Nathan E. Harms (U.S. Army Engineer Research and Development Center) Jeffrey E. Hill (UF/IFAS) Verena-Ulrike Lietze (UF/IFAS) Jennifer Russell (UF/IFAS) Emma N.I. Weeks (UF/IFAS) Marissa L. Williams (City of Maitland) External Reviewers Nancy L. Dunn (Florida LAKEWATCH volunteer) Stephen D. -

Pace University Environmental Litigation Clinic
PACE ENVIRONMENTAL LITIGATION CLINIC, INC. ELIZABETH HAUB SCHOOL OF LAW 78 NORTH BROADWAY WHITE PLAINS, NEW YORK 10603 PHONE:914.422.4343 FAX: 914.422.44437 SUPERVISING ATTORNEY ADMINISTRATOR TODD D. OMMEN JENNIFER RUHLE May, 26, 2020 Via: email ([email protected]) Mr. Kenneth Kovalchik Town of Guilderland Planning Board 5209 Western Turnpike Guilderland, NY 12084 Re: The Environmental Impact Statement for the Rapp Road Residential/Western Avenue Mixed Use Redevelopment Projects Dear Mr. Kovalchik, The Pace Environmental Litigation Clinic submits the following comments on behalf of our client, Save the Pine Bush in response to the proposed Draft Environmental Impact Statement (“EIS”) on the Rapp Road Residential/Western Avenue Mixed Use Redevelopment Projects (“The Project”) submitted to the Town of Guilderland’s Planning Board on February 19, 2020, by the project sponsor Rapp Road Development, LLC. Collectively, Commenters represent over 690 members and online activists in New York State. The project sponsor has made it painfully clear they do not care about the protecting the unique Albany Pine Bush Environment. The EIS is woefully deficient in methodology, containing almost no substantive scientific proof to support their sweeping conclusions that support their baseless claims. The project proponent on March 26, blatantly violated the SEQRA process by clear-cutting almost the entirety of site 2. This was clearly an attempt to cut down the trees on the site before the April 1, moratorium on tree cutting due to northern long-eared bat roosting. Thereby, the project proponent violated one environmental regulation to evade another environmental regulation. Lastly, it was discovered that the project sponsor did not include an important wetland report that the EIS relied on for its conclusions. -

Moths of the Kingston Study Area
Moths of the Kingston Study Area Last updated 30 July 2015 by Mike Burrell This checklist contains the 783 species known to have occurred within the Kingston Study. Major data sources include KFN bioblitzes, an earlier version created by Gary Ure (2013) and the Queen’s University Biological Station list by Kit Muma (2008). For information about contributing your sightings or to download the latest version of this checklist, please visit: http://kingstonfieldnaturalists.org/moths/moths.html Contents Superfamily: Tineoidea .................................................................................................................................................... 5 Family: Tineidae ........................................................................................................................................................... 5 Subfamily: Tineinae .................................................................................................................................................. 5 Family: Psychidae ......................................................................................................................................................... 5 Subfamily: Psychinae ................................................................................................................................................ 5 Superfamily: Gracillarioidea ............................................................................................................................................. 5 Family: Gracillariidae ................................................................................................................................................... -
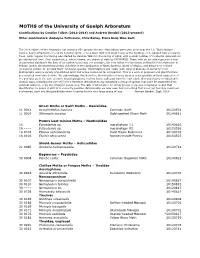
Moth Records
MOTHS of the University of Guelph Arboretum Contributions by Candice Talbot (2012-2014) and Andrew Bendall (2013-present) Other contributors: Andalyne Tofflemire, Chris Earley, Fiona Reid, Mike Kent The 2018 edition of the Arboretum list contains 851 species of moth. Most adults were seen at or near the J.C. Taylor Nature Centre, being attracted to the white building lights, or to a black light and sheet hung by the building, or to painted bait on nearby trees. Semi-regular monitoring was started by Candice Talbot in the spring of 2012, with a small number of incidental observations pre-dating that time. First record dates, where known, are shown at right as YYYYMMDD. Those with an asterisk represent a first documented sighting if the date of an earlier record was not available. We now follow the taxonomy outlined in Pohl, Patterson & Pelham (2016) Annotated taxonomic checklist of the Lepidoptera of North America, North of Mexico, and adopt their revised numbering system for all valid North American species. Identifications are made (with varying degrees of certainty) from photographs using a variety of published print and online resources for comparison. This is a work in progress and identifications are revisited from time to time. We acknowledge that definitive identification of some species is not possible without dissection of the genitalia or, in the case of some microlepidoptera, rearing larvae collected from the host plant. Our uncertainty is indicated in various ways, including the use of [t] for a tentative identification, by indicating a group of species that can't be separated from external features, or by identifying to genus only. -

Assessing Insect Biodiversity and Promoting Sustainable Practices Toward Pollinators on Campus
Sustainability Fee Project Grant Report Guidelines for grants awarded during FY2019 Due by 5pm August 1, 2019 Email pdf or word doc to [email protected] Please provide the following information in order to help the Center for Sustainability document the success of the Sustainability Fee Grant Program. Date: 1 August 2019 Name(s): Lance A. Durden, Jose A. Sanchez-Ruiz, Debra G. Albanese, Julien M. Buchbinder. Unit/Department(s): Biology E-mail address: [email protected] Phone: (912)478-5591 Project title: Assessing Insect Biodiversity and Promoting Sustainable Practices toward Pollinators on Campus. Amount granted: $27,560 Amount spent: $20,627.87 I. Project Outcomes/Value Detail the planned and actual outcomes of the project here. In a continuing effort to assess biodiversity and promote sustainable practices on the GSU (Statesboro) campus, this project aimed to add arthropods, perhaps the most biodiverse group of animals on the planet, to the vertebrate biodiversity database created in 2015. We exposed faculty, staff, students and the general public to the benefits of arthropod diversity through field activities on campus and exhibitions by focusing on charismatic insect groups and their environmental importance. Additionally, the project took advantage of the already established “iNaturalist” smartphone application to promote citizen science. We concentrated our efforts on two groups of beneficial insects in an effort to educate people of their importance, to reduce pesticide and herbicide use on campus and elsewhere, and to protect these organisms. We promoted Lepidoptera (butterflies and moths) as important pollinators of flowering plants and Odonata (dragonflies and damselflies) as important predators of biting insects such as mosquitoes and biting midges. -
University of Guelph ARBORETUM MOTHS All Species Found by CANDICE TALBOT (CT) Unless Otherwise Indicated
University of Guelph ARBORETUM MOTHS All species found by CANDICE TALBOT (CT) unless otherwise indicated. Other contributors: AB – Andrew Bendall AT – Andalyne Tofflemire CE – Chris Earley FR – Fiona Reid MK – Mike Kent Some notes about the records: Most moths were found at or near the J.C. Taylor Nature Centre, coming to building lights, or to a black light and sheet hung by the building, or to painted bait on nearby trees. Semi-regular monitoring was started by CT in 2012, with a small number of incidental observations pre- dating that time. Dates are written as 20140503 (e.g. May 3, 2014) and photographic records likely exist for all specific dates listed. Dates in brackets indicate that the moth was first recorded as present by CT before that date, but an image may not be available for the earlier sight record. Our list follows the order and groups of Beadle & Leckie (2012) Peterson Field Guide to Moths of Northeastern North America. Identifications are based on photographs using this guide as well as other published print and online resources for comparison. We acknowledge that, in some cases, definitive identification may not be possible without dissection and professional examination of the genitalia. Therefore some of our species identifications for difficult groups should be considered tentative. In other cases identification to genus is all that is possible, given the current knowledge of some groups or species complexes. Moss-Eaters - Family Micropterigidae 1. Goldcap Moss-eater Epimartyria auricrinella 20140530 AB Ghost Moths – Family Hepialidae 2. Lupulina Ghost Moth Korscheltellus lupulina 20120531 AB 3. Gold-spotted Ghost Moth Sthenopis auratus 20140621 Family Nepticulidae 4. -

Spring 2019 Bioblitz of Russ Pitman Park
RUSS PITMAN PARK SPRING BIOBLITZ - 5/6/19 – 5/7/19 Mammals: (5) Great-tailed Grackle - Quiscalus mexicanus Gray Squirrel – Sciurus carolinensis Chestnut-sided Warbler - Setophaga pensylvanica Fox Squirrel - Sciurus niger Eastern Screech Owl – Megascops asio Evening Bat - Nycticeius humeralis Purple Martin - Progne subis Eastern Red Bat - Lasiurus borealis Chuck-Will’s-Widow - Antrostomus carolinensis Big Brown Bat - Eptesicus fuscus Cedar Waxwing - Bombycilla cedrorum Carolina Wren - Thryothorus ludovicianus Birds: (23) Yellow-crowned Night Heron - Nyctanassa violacea Reptiles: (7) Downy Woodpecker - Picoides pubescens Three-toed Box Turtle – Terrapene carolina Cooper’s Hawk – Accipiter cooperii Red-eared Slider – Trachemys scripta Eastern Wood Pewee - Contopus virens Green Anole – Anolis carolinensis Wood Thrush - Hylocichla mustelina Cuban Brown Anole – Anolis sagrei Swainson’s Thrush – Catharus ustulatus Ground Skink – Scincella lateralis Chimney Swift - Chaetura pelagica Plain-bellied Water snake – Nerodia erythrogaster American Robin – Turdus migratorius Brahminy Blindsnake – indotyphlops braminus Ruby-throated Hummingbird- Archilochus colubris Northern Cardinal – Cardinalis cardinalis Amphibians: (3) Blue Jay - Cyanocitta cristata Gulf Coast Toad – Incilius nebulifer Northern Mockingbird - Mimus polyglottos Rio Grande Chirping Frog – Eleutherodactylus cystignathoides White-winged Dove - Zenaida asiatica Squirrel Treefrog – Hyla squirrela Black-bellied Whistling Duck - Dendrocygna autumnalis Rock Pigeon – Columba livia Bony Fish: -

Waterlily Leafcutter, Elophila Obliteralis (Walker) (Insecta: Lepidoptera: Crambidae: Acentropinae)1 Dale H
EENY 424 Waterlily Leafcutter, Elophila obliteralis (Walker) (Insecta: Lepidoptera: Crambidae: Acentropinae)1 Dale H. Habeck, James P. Cuda and Emma N.I. Weeks2 Introduction Acentropinae species occurring in Florida, Elophila oblite- ralis (Walker) is the most common. Although its common Hygrophila polysperma (Roxb.) T. Anderson (Polemoniales: name implies that it is a pest of waterlilies, it actually has a Acanthaceae) is a rooted submersed or emersed aquatic wide host range. Most of the damage caused by the larvae is plant in shallow water areas and saturated shorelines superficial and rarely endangers the plant, but the damage throughout Florida. This invasive aquatic plant also is observed on the hygrophila plants in this instance was known as hygrophila, hygro, East Indian hygro, green severe (Figures 1 and 2). hygro, Miramar weed, oriental ludwigia, and Indian swampweed (hereafter referred to as hygrophila). Hygrophila is a federally listed noxious weed (USDA 2012), a Florida state listed Category II prohibited plant (FDACS 2008), and a Florida Exotic Pest Plant Council Category I invasive species (FLEPPC 2019). The submersed growth habit displaces native vegetation in many canals and drain- age ditches in south Florida. The plant forms dense strands that occupy the entire water column, clogging irrigation and flood-control systems (Schmitz and Nall 1984, Sutton 1995) and interfering with navigation (Woolfe 1995). Hygrophila also creates problems as an emergent plant in some shoreline areas, including rice fields (Krombholz 1996). Figure 1. Hygrophila showing feeding damage caused by larvae of the In October 2007, we received a report from researchers waterlily leafcutter, Elophila obliteralis (Walker). at the UF/IFAS Center for Aquatic and Invasive Plants of Credits: J. -

DNA Barcoding Facilitates a Rapid Biotic Survey of a Temperate Nature Reserve
Biodiversity Data Journal 3: e6313 doi: 10.3897/BDJ.3.e6313 Taxonomic Paper Biodiversity inventories in high gear: DNA barcoding facilitates a rapid biotic survey of a temperate nature reserve Angela C Telfer‡‡§‡‡‡, Monica R Young , Jenna Quinn , Kate Perez , Crystal N Sobel , Jayme E Sones , Valerie Levesque-Beaudin‡, Rachael Derbyshire ‡, Jose Fernandez-Triana |, Rodolphe Rougerie ¶, Abinah Thevanayagam‡‡‡#‡, Adrian Boskovic , Alex V Borisenko , Alex Cadel , Allison Brown , Anais Pages¤, Anibal H Castillo ‡, Annegret Nicolai «, Barb Mockford Glenn Mockford», Belén Bukowski˄, Bill Wilson»§, Brock Trojahn , Carole Ann Lacroix˅, Chris Brimblecombe¦, Christoper Hayˀ, Christmas Ho‡, Claudia Steinke‡‡, Connor P Warne , Cristina Garrido Cortesˁ, Daniel Engelking‡‡, Danielle Wright , Dario A Lijtmaer˄, David Gascoigne», David Hernandez Martich₵, Derek Morningstar ℓ, Dirk Neumann ₰, Dirk Steinke‡, Donna DeBruin Marco DeBruin», Dylan Dobiasˁ, Elizabeth Sears‡, Ellen Richardˁ, Emily Damstra»‡, Evgeny V Zakharov , Frederic Labergeˁ, Gemma E Collins¦, Gergin A Blagoev ‡, Gerrie Grainge»‡, Graham Ansell , Greg Meredith₱, Ian Hogg¦, Jaclyn McKeown ‡, Janet Topan ‡, Jason Bracey»»‡‡‡, Jerry Guenther , Jesse Sills-Gilligan , Joseph Addesi , Joshua Persi , Kara K S Layton₳, Kareina D'Souza‡, Kencho Dorji₴, Kevin Grundy», Kirsti Nghidinwa₣, Kylee Ronnenberg‡, Kyung Min Lee₮, Linxi Xie ₦, Liuqiong Lu‡, Lyubomir Penev₭, Mailyn Gonzalez ₲, Margaret E Rosati ‽, Mari Kekkonen‡‡‡, Maria Kuzmina , Marianne Iskandar , Marko Mutanen₮, Maryam Fatahi‡, Mikko Pentinsaari₮, -

Moth Records
MOTHS of the University of Guelph Arboretum Contributions by Candice Talbot (2012-2014) and Andrew Bendall (2013-present) Other contributors: Andalyne Tofflemire, Chris Early, Fiona Reid, Mike Kent The 2016 edition of the Arboretum list contains 844 species of moth. Most adults were seen at or near the J.C. Taylor Nature Centre, being attracted to the white building lights, or to a black light and sheet hung by the building, or to painted bait on nearby trees. Semi-regular monitoring was started by Candice Talbot in the spring of 2012, with a small number of incidental observations pre-dating that time. First record dates, where known, are shown at right as YYYYMMDD. Those with an asterisk represent AB's first documented sighting if the date of an earlier record was not available. Starting with this version of the list I've followed the taxonomy outlined in Pohl, Patterson & Pelham (2016) Annotated taxonomic checklist of the Lepidoptera of North America, North of Mexico, and adopt their revised numbering system for all valid North American species. Identifications are made (with varying degrees of certainty) from photographs using a variety of published print and online resources for comparison. This is a work in progress and identifications are revisited from time to time. We acknowledge that definitive identification of some species is not possible without dissection of the genitalia or, in the case of some microlepidoptera, rearing larvae collected from the host plant. Our uncertainty is indicated in various ways, including the use of [t] for a tentative identification, by indicating a pair of species that can't be separated from external features, or by identifying to genus only.