2014 Hydrilla Integrated Management
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Red Names=Invasive Species Green Names=Native Species
CURLY-LEAF PONDWEED EURASIAN WATERMIL- FANWORT CHARA (Potamogeton crispus) FOIL (Cabomba caroliniana) (Chara spp.) This undesirable exotic, also known (Myriophyllum spicatum) This submerged exotic Chara is typically found growing in species is not common as Crisp Pondweed, bears a waxy An aggressive plant, this exotic clear, hard water. Lacking true but management tools are cuticle on its upper leaves making milfoil can grow nearly 10 feet stems and leaves, Chara is actually a limited. Very similar to them stiff and somewhat brittle. in length forming dense mats form of algae. It’s stems are hollow aquarium species. Leaves The leaves have been described as at the waters surface. Grow- with leaf-like structures in a whorled are divided into fine resembling lasagna noodles, but ing in muck, sand, or rock, it pattern. It may be found growing branches in a fan-like ap- upon close inspection a row of has become a nuisance plant with tiny, orange fruiting bodies on pearance, opposite struc- “teeth” can be seen to line the mar- in many lakes and ponds by the branches called akinetes. Thick ture, spanning 2 inches. gins. Growing in dense mats near quickly outcompeting native masses of Chara can form in some Floating leaves are small, the water’s surface, it outcompetes species. Identifying features areas. Often confused with Starry diamond shape with a native plants for sun and space very include a pattern of 4 leaves stonewort, Coontail or Milfoils, it emergent white/pinkish early in spring. By midsummer, whorled around a hollow can be identified by a gritty texture flower. -

Hydrilla Vs. New York
1 comicvine.com Hydrilla vs. New York UMISC October 16, 2018 2 Hydrilla in New York High priority species prohibited by Part 575 Now found at 32 locations throughout New York Often found near boat launches DeviantArt Waterfowl also considered a vector 3 Hydrilla in New York First discovered in 2008 2008 - Creamery Pond, Orange County 2008 – Sans Souci Lake, Lotus Lake, Suffolk County 2009 - Lake Ronkonkoma, Blydenburgh/New Mill Pond, Phillips Mill Pond, Suffolk County 2009 – Frost Mill Pond, Suffolk County 2011- Smith Pond, Great Patchoque Lake, Suffolk County; Cayuga Inlet, Tompkins County 2012 – several private ponds, Broome County 2012 – Cayuga Lake, Tompkins County; Tonawanda/Erie Canal, Niagara and Erie Counties 2013 – Croton River, Westchester County 2013 – Millers Pond, Suffolk County; Unnamed pond, Tioga County 2014 – New Croton Reservoir, Westchester County 2014 – Prospect Park, Brooklyn, Kings County 2015 – Tinker Nature Park pond, Monroe County 2016 – Aurora (Cayuga Lake), Tompkins County 2016 – Spencer Pond, Tioga County 2016 – Halsey Neck Road Pond, Suffolk County 2018 - Kuhlman Pond, Tioga County 2018 - Avon Pond, Frank Melville Pond, and East Setauket, Suffolk County 2018 – Allison Pond, Staten Island, Richmond County 4 Management Options in Place 1) No management 2) Benthic mats 3) Triploid Grass Carp 4) Herbicide 5) Combination (IPM) 5 Option: No management Suffolk County: • Lake Ronkonkoma (10 acres of 240 acres) • Sans Souci (southern 5 acres) • Lotus Lake (13 acres) • Blydenburgh/New Mill Pond (110 acres, coverage -

Integrated Pest Management: Current and Future Strategies
Integrated Pest Management: Current and Future Strategies Council for Agricultural Science and Technology, Ames, Iowa, USA Printed in the United States of America Cover design by Lynn Ekblad, Different Angles, Ames, Iowa Graphics and layout by Richard Beachler, Instructional Technology Center, Iowa State University, Ames ISBN 1-887383-23-9 ISSN 0194-4088 06 05 04 03 4 3 2 1 Library of Congress Cataloging–in–Publication Data Integrated Pest Management: Current and Future Strategies. p. cm. -- (Task force report, ISSN 0194-4088 ; no. 140) Includes bibliographical references and index. ISBN 1-887383-23-9 (alk. paper) 1. Pests--Integrated control. I. Council for Agricultural Science and Technology. II. Series: Task force report (Council for Agricultural Science and Technology) ; no. 140. SB950.I4573 2003 632'.9--dc21 2003006389 Task Force Report No. 140 June 2003 Council for Agricultural Science and Technology Ames, Iowa, USA Task Force Members Kenneth R. Barker (Chair), Department of Plant Pathology, North Carolina State University, Raleigh Esther Day, American Farmland Trust, DeKalb, Illinois Timothy J. Gibb, Department of Entomology, Purdue University, West Lafayette, Indiana Maud A. Hinchee, ArborGen, Summerville, South Carolina Nancy C. Hinkle, Department of Entomology, University of Georgia, Athens Barry J. Jacobsen, Department of Plant Sciences and Plant Pathology, Montana State University, Bozeman James Knight, Department of Animal and Range Science, Montana State University, Bozeman Kenneth A. Langeland, Department of Agronomy, University of Florida, Institute of Food and Agricultural Sciences, Gainesville Evan Nebeker, Department of Entomology and Plant Pathology, Mississippi State University, Mississippi State David A. Rosenberger, Plant Pathology Department, Cornell University–Hudson Valley Laboratory, High- land, New York Donald P. -

Introduction to Common Native & Invasive Freshwater Plants in Alaska
Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska Cover photographs by (top to bottom, left to right): Tara Chestnut/Hannah E. Anderson, Jamie Fenneman, Vanessa Morgan, Dana Visalli, Jamie Fenneman, Lynda K. Moore and Denny Lassuy. Introduction to Common Native & Potential Invasive Freshwater Plants in Alaska This document is based on An Aquatic Plant Identification Manual for Washington’s Freshwater Plants, which was modified with permission from the Washington State Department of Ecology, by the Center for Lakes and Reservoirs at Portland State University for Alaska Department of Fish and Game US Fish & Wildlife Service - Coastal Program US Fish & Wildlife Service - Aquatic Invasive Species Program December 2009 TABLE OF CONTENTS TABLE OF CONTENTS Acknowledgments ............................................................................ x Introduction Overview ............................................................................. xvi How to Use This Manual .................................................... xvi Categories of Special Interest Imperiled, Rare and Uncommon Aquatic Species ..................... xx Indigenous Peoples Use of Aquatic Plants .............................. xxi Invasive Aquatic Plants Impacts ................................................................................. xxi Vectors ................................................................................. xxii Prevention Tips .................................................... xxii Early Detection and Reporting -

Natural Resource Inventory Smith-Sargent
NATURAL RESOURCE INVENTORY of the SMITH-SARGENT ROAD PROPERTY Holderness, NH FINAL REPORT [Smith-Sargent Property Upper Marsh as seen from south boundary] Compiled by: Dr. Rick Van de Poll Ecosystem Management Consultants 30 N. Sandwich Rd. Center Sandwich, NH 03227 603-284-6851 [email protected] Submitted to: Holderness Conservation Commission June 30, 2016 i SUMMARY Between October 2015 and June 2016 a comprehensive natural resources inventory (NRI) was completed by Ecosystem Management Consultants (EMC) of Sandwich, NH on the 8.5-acre town conservation land at the corner of Sargent Road and Smith Road in Holderness, NH. Managed by the Holderness Conservation Commission (HCC), this parcel was obtained largely for the complex wetland system that occupies more than 65% of the parcel. The purpose of the NRI was to inform the town about the qualities of the natural resources on the lot, as well as to determine whether or not the site would be suitable for limited environmental education for the general public. Three site visits were conducted at the Sargent-Smith Road Property for the purpose of gathering NRI data. A fourth visit was also made on November 15, 2015 for the purpose of educating the HCC and other town officials about the extent and functional value of the wetlands on the parcel. The first field visit in October provided an initial review of the location of the parcel, the boundary of the wetland, and the plant and animal resources present. A second site visit in January was held for the purpose of tracking mammals during good snow cover. -

Hydrilla Fact Sheet
HHEADINGEADING OFFOFF HHYDRILLAYDRILLA Another invasive species is heading towards the Great Lakes: Hydrilla verticillata onnative species are a great threat to the Great Because it is so invasive and pervasive, hydrilla Lakes region. So far, much focus has been greatly disrupts the ecological balance of all the areas Nplaced on sea lamprey, zebra mussels, alewife, where it grows. Large, dense hydrilla mats inhibit spiny water fleas, ruffe, goby, purple loosestrife and sunlight from penetrating the water and shade out native Eurasian watermilfoil. Soon, another species - Hydrilla plant species that live in the waters below them. Because verticillata – may join this list. hydrilla mats slow the movement of water, sediments Hydrilla is a robust aquatic plant that may survive build up where hydrilla thrives, decreasing the turbidity and thrive in waters of this area. As of December 2005, and creating good breeding grounds for mosquitoes. biologists have found no evidence of hydrilla in Michigan’s shallow Great Economic Impacts Lakes bays, 11,000 inland lakes or Hydrilla has serious economic thousands of miles of streams. effects resulting from the ecological However, the level of concern for impacts. As mentioned before, hydrilla ecological damage and economic harm can slow the movement of water, to Michigan’s water resources has disrupting the water supply, impeding increased due to the fact that hydrilla drainage and irrigation. This adds costs is now known to exist in two Great to the agricultural economy and also Lakes states, Pennsylvania and New negatively affects real estate values that York. are dependent upon attractive nearby waterways. Ecological Impacts Hydrilla also affects recreational Hydrilla has many adaptive qualities activities and the associated economy. -

Download This Article in PDF Format
Knowl. Manag. Aquat. Ecosyst. 2018, 419, 42 Knowledge & © K. Pabis, Published by EDP Sciences 2018 Management of Aquatic https://doi.org/10.1051/kmae/2018030 Ecosystems www.kmae-journal.org Journal fully supported by Onema REVIEW PAPER What is a moth doing under water? Ecology of aquatic and semi-aquatic Lepidoptera Krzysztof Pabis* Department of Invertebrate Zoology and Hydrobiology, University of Lodz, Banacha 12/16, 90-237 Lodz, Poland Abstract – This paper reviews the current knowledge on the ecology of aquatic and semi-aquatic moths, and discusses possible pre-adaptations of the moths to the aquatic environment. It also highlights major gaps in our understanding of this group of aquatic insects. Aquatic and semi-aquatic moths represent only a tiny fraction of the total lepidopteran diversity. Only about 0.5% of 165,000 known lepidopterans are aquatic; mostly in the preimaginal stages. Truly aquatic species can be found only among the Crambidae, Cosmopterigidae and Erebidae, while semi-aquatic forms associated with amphibious or marsh plants are known in thirteen other families. These lepidopterans have developed various strategies and adaptations that have allowed them to stay under water or in close proximity to water. Problems of respiratory adaptations, locomotor abilities, influence of predators and parasitoids, as well as feeding preferences are discussed. Nevertheless, the poor knowledge on their biology, life cycles, genomics and phylogenetic relationships preclude the generation of fully comprehensive evolutionary scenarios. Keywords: Lepidoptera / Acentropinae / caterpillars / freshwater / herbivory Résumé – Que fait une mite sous l'eau? Écologie des lépidoptères aquatiques et semi-aquatiques. Cet article passe en revue les connaissances actuelles sur l'écologie des mites aquatiques et semi-aquatiques, et discute des pré-adaptations possibles des mites au milieu aquatique. -

An Abstract of the Thesis Of
AN ABSTRACT OF THE THESIS OF Kathryn Wellons for the degree of Master of Science in Horticulture presented on June 7, 2018. Title: Ecophenology and Control of European Frogbit in a Hybrid Cattail Marsh of the St. Marys River, Michigan. Abstract approved: _____________________________________________________________________ Dennis Albert Great Lakes coastal wetland communities are threatened by the impacts of invasive plants on ecosystem function and biodiversity. What allows invasive plants to become dominant in invaded communities can be hard to define and context-dependent. Traits associated with invasion success in wetland systems – rapid vegetative growth, competitive superiority in resource acquisition, and tolerance for high nutrient levels – are shared by two co-occurring invasive plants, hybrid cattail (Typha × glauca) and European frogbit (Hydrocharis morsus- ranae). European frogbit is a free-floating weed causing substantial negative impacts to native ecosystems in the Great Lakes region. It is thought to be facilitated by the presence of emergent plants like hybrid cattail, but the nature of this relationship has not been empirically demonstrated or utilized in management strategies. The purpose of this thesis was to advance understanding of the phenology, ecology, and control of European frogbit within an invaded hybrid cattail marsh along the St. Marys River, a connecting channel between Lakes Huron and Superior. This marsh was a valuable site both for investigating the relationship between hybrid cattail and European frogbit and for assessing the role of deep water in the development and control of European frogbit. In an observational study, measures of the phenological development of European frogbit were accompanied by measures of environmental variables and estimates of plant community abundances to explore associations between European frogbit development and environmental conditions during a high-water period in the Great Lakes. -
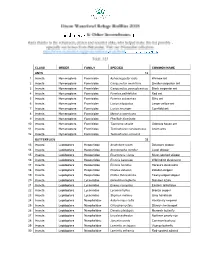
Class Order Family Species Common Name Ants 12 1
CLASS ORDER FAMILY SPECIES COMMON NAME ANTS 12 1 Insecta Hymenoptera Formicidae Aphaenogaster rudis Winnow ant 2 Insecta Hymenoptera Formicidae Camponotus nearcticus Smaller carpenter ant 3 Insecta Hymenoptera Formicidae Camponotus pennsylvanicus Black carpenter ant 4 Insecta Hymenoptera Formicidae Formica pallidefulva Red ant 5 Insecta Hymenoptera Formicidae Formica subsericea Silky ant 6 Insecta Hymenoptera Formicidae Lasius interjectus Larger yellow ant 7 Insecta Hymenoptera Formicidae Lasius neoniger Cornfield ant 8 Insecta Hymenoptera Formicidae Myrmica americana 9 Insecta Hymenoptera Formicidae Pheidole bicarinata 10 Insecta Hymenoptera Formicidae Tapinoma sessile Odorous house ant 11 Insecta Hymenoptera Formicidae Temnothorax curvispinosus Acorn ants 12 Insecta Hymenoptera Formicidae Temnothorax schaumii BUTTERFLIES 32 13 Insecta Lepidoptera Hesperiidae Anatrytone logan Delaware skipper 14 Insecta Lepidoptera Hesperiidae Ancyloxypha numitor Least skipper 15 Insecta Lepidoptera Hesperiidae Epargyreus clarus Silver-spotted skipper 16 Insecta Lepidoptera Hesperiidae Erynnis baptisiae Wild indigo duskywing 17 Insecta Lepidoptera Hesperiidae Erynnis horatius Horace's duskywing 18 Insecta Lepidoptera Hesperiidae Poanes zabulon Zabulon skipper 19 Insecta Lepidoptera Hesperiidae Polites themistocles Tawny-edged skipper 20 Insecta Lepidoptera Lycaenidae Celastrina neglecta Summer azure 21 Insecta Lepidoptera Lycaenidae Everes comyntas Eastern tailed blue 22 Insecta Lepidoptera Lycaenidae Lycaena hyllus Bronze copper 23 Insecta Lepidoptera -

Folivory and Disease Occurrence on Ludwigia Hexapetala in Guntersville Reservoir, Alabama
J. Aquat. Plant Manage. 55: 19–25 Folivory and disease occurrence on Ludwigia hexapetala in Guntersville Reservoir, Alabama NATHAN E. HARMS, JUDY F. SHEARER, AND MICHAEL J. GRODOWITZ* ABSTRACT southeastern United States, with disjunct populations in California and Oregon (Grewell et al. 2016). Invasive We report leaf feeding, disease occurrence, and associ- populations also exist outside the United States in France, ated indigenous herbivore/fungal pathogen communities Belgium, Italy, Spain, Greece, the United Kingdom, and The on the introduced wetland species Ludwigia hexapetala at Netherlands (Dandelot et al. 2005, Thouvenot et al. 2013). Guntersville Reservoir, AL. Plant populations were sam- Closely related Ludwigia are difficult to distinguish mor- pled on three dates from May to September 2014. A phologically, and conflicting diagnostic characters have complex of indigenous herbivore and fungal taxa, mostly been presented by various authors (Nesom and Kartesz known from other Ludwigia spp., resulted in peak feeding 2000). Ludwigia hexapetala is decaploid (2n ¼ 80; Zardini et al. and disease occurrence on 88% and 92% of sampled 1991), a characteristic that may contribute to relative leaves, respectively. Herbivore damage declined over the invasiveness over other Ludwigia spp. (Pandit et al. 2011, growing season from 78 to 21% of sampled leaves, and Grewell et al. 2016). disease symptom occurrence increased from 0 to 80%. Management of L. hexapetala in the United States is a Total leaf damage (percent leaf area) from both herbivory concern as the number and distribution of infestations and disease was determined by software image analyses of increase. Ludwigia hexapetala causes economic damage floating and aerial leaves and reached 14% total reduction through disruption of flood control, irrigation water in photosynthetic tissues by September 2014. -

Download the NOAA Fact Nonindigenous Aquatic Nuisance Prevention and Sheet from the Public Domain
Reviewed March 2020 Publishing Information The University of Florida Institute of Food and Agricultural Sciences (UF/IFAS) is an Equal Opportunity Institution. UF/IFAS is committed to diversity of people, thought and opinion, to inclusiveness and to equal opportunity. The use of trade names in this publication is solely for the purpose of providing specific information. UF/IFAS does not guarantee or warranty the products named, and references to them in this publication do not signify our approval to the exclusion of other products of suitable composition. All chemicals should be used in accordance with directions on the manufacturer’s label. Use pesticides and herbicides safely. Read and follow directions on the manufacturer’s label. For questions about using pesticides, please contact your local county Extension office. Visit http://solutionsforyourlife.ufl.edu/map to find an office near you. Copyright 2014, The University of Florida Editors Jennifer L. Gillett-Kaufman (UF/IFAS) Verena-Ulrike Lietze (UF/IFAS) Emma N.I. Weeks (UF/IFAS) Contributing Authors Julie Baniszewski (UF/IFAS) Ted D. Center (USDA/ARS, retired) Byron R. Coon (Argosy University) James P. Cuda (UF/IFAS) Amy L. Giannotti (City of Winter Park) Judy L. Gillmore (UF/IFAS) Michael J. Grodowitz (U.S. Army Engineer Research and Development Center) Dale H. Habeck, deceased (UF/IFAS) Nathan E. Harms (U.S. Army Engineer Research and Development Center) Jeffrey E. Hill (UF/IFAS) Verena-Ulrike Lietze (UF/IFAS) Jennifer Russell (UF/IFAS) Emma N.I. Weeks (UF/IFAS) Marissa L. Williams (City of Maitland) External Reviewers Nancy L. Dunn (Florida LAKEWATCH volunteer) Stephen D. -

Quelques Papillons De Nuit De La Réserve Faunique De Matane
Quelques papillons de nuit de la réserve faunique de Matane Le mont Blanc à l’arrière-plan Comité de protection des monts Chic-Chocs Rapport produit par Jacques Larivée Rimouski, mai 2017 Papillons de nuit de la réserve faunique de Matane C’est à l’invitation du Comité de protection des monts Chic-Chocs que je joins du 15 au 17 août 2016 un groupe de naturalistes qui a pour objectif d’améliorer les connaissances de la flore et de la faune du territoire de la réserve faunique de Matane. Voici les membres de l’équipe dans l’ordre où ils apparaissent sur la photo. Photo Claude Gauthier Claude Gauthier (ornithologie, photographie, kayak, transport et sécurité) ; Christian Grenier (botanique des plantes vasculaires et photographie) ; Pierre Fradette (organisation, ornithologie, photographie, transport et sécurité), Louis Fradette (organisation, ornithologie, photographie, kayak, transport et sécurité) ; Pierre Lévesque (bryologie et photographie) ; Jean Faubert (bryologie et photographie) ; Jacques Larivée (ornithologie, photographie et entomologie) ; Gaétan Caron (organisation, transport, connaissance du territoire et sécurité). 2 Papillons de nuit de la réserve faunique de Matane Mon « travail » consiste à noter mes observations des oiseaux le jour partout sur le territoire, comme le font les autres ornithologues, et à photographier les papillons de nuit le soir à l’Étang à la Truite le 15 et le 17 août et au sommet du mont Blanc le soir du 16 août. Mes observations d’oiseaux sont enregistrées sur eBird et sont résumées dans ce document. Le texte qui suit présente 3 listes d’espèces incluant au moins une photo par espèce : la liste des papillons de nuit photographiés à l’Étang à la Truite suivie de la liste des papillons de nuit photographiés au mont Blanc et de la liste des papillons de nuit non identifiés.