The Ryanodine Receptor As a Sensor for Cellular Environments in Muscles
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unnatural Verticilide Enantiomer Inhibits Type 2 Ryanodine Receptor-Mediated Calcium Leak and Is Antiarrhythmic
Unnatural verticilide enantiomer inhibits type 2 ryanodine receptor-mediated calcium leak and is antiarrhythmic Suzanne M. Batistea,1, Daniel J. Blackwellb,1, Kyungsoo Kimb,1, Dmytro O. Kryshtalb, Nieves Gomez-Hurtadob, Robyn T. Rebbeckc, Razvan L. Corneac, Jeffrey N. Johnstona,2, and Bjorn C. Knollmannb,2 aDepartment of Chemistry, Vanderbilt University, Nashville, TN 37235; bDepartment of Medicine, Vanderbilt University Medical Center, Nashville, TN 37232; and cDepartment of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, Minneapolis, MN 55455 Edited by Dale L. Boger, The Scripps Research Institute, La Jolla, CA, and approved January 15, 2019 (received for review September 27, 2018) Ca2+ leak via ryanodine receptor type 2 (RyR2) can cause poten- heart diseases associated with both atrial and ventricular arrhyth- tially fatal arrhythmias in a variety of heart diseases and has also mia (9). Mutations in RyR2 and its binding partners, which increase + been implicated in neurodegenerative and seizure disorders, mak- SR Ca2 leak, cause primary atrial and ventricular arrhythmia ing RyR2 an attractive therapeutic target for drug development. syndromes such as catecholaminergic polymorphic ventricular Here we synthesized and investigated the fungal natural product tachycardia (CPVT), providing strong evidence for the mechanistic and known insect RyR antagonist (−)-verticilide and several conge- contribution of RyR2 to arrhythmia risk in humans (10). Further ners to determine their activity against mammalian RyR2. Although support comes from gene-targeted mouse models of CPVT, where + the cyclooligomeric depsipeptide natural product (−)-verticilide had catecholamine-induced spontaneous Ca2 release from the SR no effect, its nonnatural enantiomer [ent-(+)-verticilide] signifi- via RyR2 generates potentially fatal cardiac arrhythmias (11, 12). -

Association of Serum S100B, S100A1 and Zinc-Α2
Progress in Nutrition 2019; Vol. 21, Supplement 1: 154-162 DOI: 10.23751/pn.v21i1-S.5846 © Mattioli 1885 Original articles Association of serum S100B, S100A1 and Zinc-α2- Glycoprotein levels with anthropometric, metabolic and clinical indices in men and women Sorayya Kheirouri1, Mohammad Alizadeh1, Elham Ebrahimi2, Masoumeh Jabbari2 1Associate Professor, Department of Nutrition, Tabriz University of Medical Sciences, Tabriz, Iran - Email: kheirouris@tbzmed. ac.ir, [email protected]; 2MSc. student, Department of Nutrition, Tabriz University of Medical Sciences, Tabriz, Iran Summary. Objectives: We aimed to investigate serum levels of S100B, S100A1, and Zinc-α2- glycoprotein (ZAG) in men and women and to find association of these proteins with anthropometric, metabolic and clini- cal indices. Methods: Eighty-eight apparently healthy adults, 43 men and 45 women, participated in the study. The participants’ body mass index (BMI), waist circumference (WC), systolic and diastolic blood pressure (SBP and DBP) were measured. Serum levels of total cholesterol (TC), triglyceride (TG), low and high den- sity lipoprotein cholesterol (LDL-C and HDL-C), fasting blood sugar (FBS), insulin, S100B, S100A1 and ZAG protein were examined by enzymatic and ELISA laboratory methods. Homeostatic model assessment- insulin resistance (HOMA-IR) index was calculated. Results: Serum levels of S100B, S100A1 and ZAG were comparable between men and women groups. S100B protein was positively associated with TG (r= 0.41, p= 0.006), SBP (r= 0.46, p= 0.002), and DBP (r= 0.37, p= 0.02), but negatively with HDL-c in men. Serum levels of S100A1 were significantly and negatively correlated with WC (r= -0.33, p= 0.03), TG (r= -0.37, p= 0.01), insulin (r= -0.31, p= 0.04) and HOMA-IR (r= -0.32, p= 0.03), in women. -

Aquaporin Channels in the Heart—Physiology and Pathophysiology
International Journal of Molecular Sciences Review Aquaporin Channels in the Heart—Physiology and Pathophysiology Arie O. Verkerk 1,2,* , Elisabeth M. Lodder 2 and Ronald Wilders 1 1 Department of Medical Biology, Amsterdam University Medical Centers, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] 2 Department of Experimental Cardiology, Amsterdam University Medical Centers, University of Amsterdam, 1105 AZ Amsterdam, The Netherlands; [email protected] * Correspondence: [email protected]; Tel.: +31-20-5664670 Received: 29 March 2019; Accepted: 23 April 2019; Published: 25 April 2019 Abstract: Mammalian aquaporins (AQPs) are transmembrane channels expressed in a large variety of cells and tissues throughout the body. They are known as water channels, but they also facilitate the transport of small solutes, gasses, and monovalent cations. To date, 13 different AQPs, encoded by the genes AQP0–AQP12, have been identified in mammals, which regulate various important biological functions in kidney, brain, lung, digestive system, eye, and skin. Consequently, dysfunction of AQPs is involved in a wide variety of disorders. AQPs are also present in the heart, even with a specific distribution pattern in cardiomyocytes, but whether their presence is essential for proper (electro)physiological cardiac function has not intensively been studied. This review summarizes recent findings and highlights the involvement of AQPs in normal and pathological cardiac function. We conclude that AQPs are at least implicated in proper cardiac water homeostasis and energy balance as well as heart failure and arsenic cardiotoxicity. However, this review also demonstrates that many effects of cardiac AQPs, especially on excitation-contraction coupling processes, are virtually unexplored. -

Supplemental Material
Supplemental Table B ARGs in alphabetical order Symbol Title 3 months 6 months 9 months 12 months 23 months ANOVA Direction Category 38597 septin 2 1557 ± 44 1555 ± 44 1579 ± 56 1655 ± 26 1691 ± 31 0.05219 up Intermediate 0610031j06rik kidney predominant protein NCU-G1 491 ± 6 504 ± 14 503 ± 11 527 ± 13 534 ± 12 0.04747 up Early Adult 1G5 vesicle-associated calmodulin-binding protein 662 ± 23 675 ± 17 629 ± 16 617 ± 20 583 ± 26 0.03129 down Intermediate A2m alpha-2-macroglobulin 262 ± 7 272 ± 8 244 ± 6 290 ± 7 353 ± 16 0.00000 up Midlife Aadat aminoadipate aminotransferase (synonym Kat2) 180 ± 5 201 ± 12 223 ± 7 244 ± 14 275 ± 7 0.00000 up Early Adult Abca2 ATP-binding cassette, sub-family A (ABC1), member 2 958 ± 28 1052 ± 58 1086 ± 36 1071 ± 44 1141 ± 41 0.05371 up Early Adult Abcb1a ATP-binding cassette, sub-family B (MDR/TAP), member 1A 136 ± 8 147 ± 6 147 ± 13 155 ± 9 185 ± 13 0.01272 up Midlife Acadl acetyl-Coenzyme A dehydrogenase, long-chain 423 ± 7 456 ± 11 478 ± 14 486 ± 13 512 ± 11 0.00003 up Early Adult Acadvl acyl-Coenzyme A dehydrogenase, very long chain 426 ± 14 414 ± 10 404 ± 13 411 ± 15 461 ± 10 0.01017 up Late Accn1 amiloride-sensitive cation channel 1, neuronal (degenerin) 242 ± 10 250 ± 9 237 ± 11 247 ± 14 212 ± 8 0.04972 down Late Actb actin, beta 12965 ± 310 13382 ± 170 13145 ± 273 13739 ± 303 14187 ± 269 0.01195 up Midlife Acvrinp1 activin receptor interacting protein 1 304 ± 18 285 ± 21 274 ± 13 297 ± 21 341 ± 14 0.03610 up Late Adk adenosine kinase 1828 ± 43 1920 ± 38 1922 ± 22 2048 ± 30 1949 ± 44 0.00797 up Early -
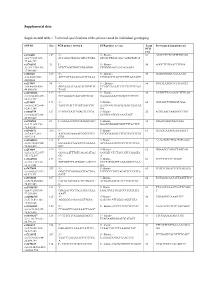
Supplemental Data Supplemental Table 1. Technical Specifications of the Primers Used for Individual Genotyping
Supplemental data Supplemental table 1. Technical specifications of the primers used for individual genotyping SNP ID Size PCR primer forward PCR primer reverse Temp Pyrosequencing primer(s) PCR (°C) rs776108 127 5’- 5’- Biotin- 61 ATACCTCTCATTTTGCAG chr3:77,825,927- ACCAGGCTAGGCATGCTATA GTCACTTAACAGCAGTGTGTCA 77,826,729 rs3746192 92 5’- 5’- Biotin- 56 AGGCTTGTAACCTGGA chr19:17,946,368- ATTCTAGGTGGCATGAGGG CTGGGGAGCAACAGAAGA 17,946,488 rs988147 169 5' - 5' - Biotine- 53 GCAGGGGGTAGAAATG chr6:45282108- AGCCATTAAAGAATTTCAAA TTGGATTTTATTCTTGTAATAGG 45282608 rs227849 94 5' - 5' - Biotine- 56 GGTTTAAGGTCTTTGCAT chr6:44,806,436- AGGAAAATAAACATGTGGTT TCTACCAATATTTTCTTTCGTAG 44,806,936 TAAG T rs10733833 127 5' - 5' - Biotine- 58 CATGTTTAAAACCTTTCAG chr10:68,418,227- GCCAAAACCAACAGTTCAT GAAAAAAATTGCACCTGTCTC 68,418,727 rs322609 197 5’- 5’-Biotin- 60 GGTAGCTGTGGGTGGA chr16:62,432,604- TAGTTGATTTTGCCAACCTG AAATGGGTGACAGAAGTAATAA 62,433,104 GA rs1884779 127 5’-TGGCTATTGGAGTTCTCA 5’-Biotin- 55 AGTGAATTAAGGGCTTGT chr20:45,857,969- CCATCCATCCCAAATAGT 45,858,469 rs4703908 83 5’- GAAAATGCCCAAGGTGAC 5’-Biotin- 52 GTGACAGTGGGCAAA chr5:71,802,353- GAATGTGGGTGTGTTTTACTCT 71,802,853 rs6946871 290 5’- 5’-Biotin- 61 GGAGGAAAGGAAAAGTT chr7:4,037,310- ATCAGATAAAATCGGCTTCT TCGGGAAGGTTTTTGTACTTTTG 4,037,810 GTG rs11865033 123 5’- 5’-Biotin- 61 AAAGTCTCTTCCTATGAGC chr16:78,082,945- AATAAACCAAGCCCTGAAAA ACTAAAATCCCCCTTTCCTCCA 78,083,445 GTC rs247004 170 5’- 5’-Biotin- 62 GGAAGCCAGACTAGCAG chr5:131,372,007- GGGGAATTTGTCAGAGATAG GGGATCCTCTACCATCCAAATA 131,372,507 GG -

Glial Protein S100B Modulates Long-Term Neuronal Synaptic Plasticity
Glial protein S100B modulates long-term neuronal synaptic plasticity Hiroshi Nishiyama*†, Thomas Kno¨ pfel†, Shogo Endo‡, and Shigeyoshi Itohara*§ *Laboratories for Behavioral Genetics and †Neuronal Circuit Dynamics, and ‡Neuronal Circuit Mechanisms Research Group, Brain Science Institute (BSI), Institute of Physical and Chemical Research (RIKEN), 2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan Communicated by Richard F. Thompson, University of Southern California, Los Angeles, CA, January 11, 2002 (received for review August 1, 2001) Glial cells are traditionally regarded as elements for structural subject of debate (1). Transgenic mice overexpressing human support and ionic homeostasis, but have recently attracted atten- S100B exhibit impaired hippocampal LTP and spatial learning tion as putative integral elements of the machinery involved in (11). Transgenic mice overexpressing S100B might not be ap- synaptic transmission and plasticity. Here, we demonstrate that propriate for evaluating the physiological roles of S100B, how- calcium-binding protein S100B, which is synthesized in consider- ever, because overexpression of S100B partly mimics patholog- able amounts in astrocytes (a major glial cell subtype), modulates ical conditions in some neuronal diseases, such as Down’s long-term synaptic plasticity. Mutant mice devoid of S100B devel- syndrome and Alzheimer’s disease (12, 13). The constitutive oped normally and had no detectable abnormalities in the cyto- overexpression of S100B might cause chronic neuronal damage architecture of the brain. These mutant mice, however, had (14, 15). Thus, there is no clear consensus regarding the signif- strengthened synaptic plasticity as identified by enhanced long- icance of this glial protein in neuronal synaptic plasticity. term potentiation (LTP) in the hippocampal CA1 region. -

Ion Channels 3 1
r r r Cell Signalling Biology Michael J. Berridge Module 3 Ion Channels 3 1 Module 3 Ion Channels Synopsis Ion channels have two main signalling functions: either they can generate second messengers or they can function as effectors by responding to such messengers. Their role in signal generation is mainly centred on the Ca2 + signalling pathway, which has a large number of Ca2+ entry channels and internal Ca2+ release channels, both of which contribute to the generation of Ca2 + signals. Ion channels are also important effectors in that they mediate the action of different intracellular signalling pathways. There are a large number of K+ channels and many of these function in different + aspects of cell signalling. The voltage-dependent K (KV) channels regulate membrane potential and + excitability. The inward rectifier K (Kir) channel family has a number of important groups of channels + + such as the G protein-gated inward rectifier K (GIRK) channels and the ATP-sensitive K (KATP) + + channels. The two-pore domain K (K2P) channels are responsible for the large background K current. Some of the actions of Ca2 + are carried out by Ca2+-sensitive K+ channels and Ca2+-sensitive Cl − channels. The latter are members of a large group of chloride channels and transporters with multiple functions. There is a large family of ATP-binding cassette (ABC) transporters some of which have a signalling role in that they extrude signalling components from the cell. One of the ABC transporters is the cystic − − fibrosis transmembrane conductance regulator (CFTR) that conducts anions (Cl and HCO3 )and contributes to the osmotic gradient for the parallel flow of water in various transporting epithelia. -

Disease Mutations in the Ryanodine Receptor N-Terminal Region Couple to a Mobile Intersubunit Interface
ARTICLE Received 1 Oct 2012 | Accepted 15 Jan 2013 | Published 19 Feb 2013 DOI: 10.1038/ncomms2501 OPEN Disease mutations in the ryanodine receptor N-terminal region couple to a mobile intersubunit interface Lynn Kimlicka1, Kelvin Lau1, Ching-Chieh Tung1 & Filip Van Petegem1 Ryanodine receptors are large channels that release Ca2 þ from the endoplasmic and sar- coplasmic reticulum. Hundreds of RyR mutations can cause cardiac and skeletal muscle disorders, yet detailed mechanisms explaining their effects have been lacking. Here we compare pseudo-atomic models and propose that channel opening coincides with widen- ing of a cytoplasmic vestibule formed by the N-terminal region, thus altering an interface targeted by 20 disease mutations. We solve crystal structures of several disease mutants that affect intrasubunit domain–domain interfaces. Mutations affecting intrasubunit ionic pairs alter relative domain orientations, and thus couple to surrounding interfaces. Buried disease mutations cause structural changes that also connect to the intersubunit contact area. These results suggest that the intersubunit contact region between N-terminal domains is a prime target for disease mutations, direct or indirect, and we present a model whereby ryanodine receptors and inositol-1,4,5-trisphosphate receptors are activated by altering domain arrangements in the N-terminal region. 1 Department of Biochemistry and Molecular Biology, Life Sciences Institute, University of British Columbia, Vancouver, British Columbia, Canada V6T 1Z3. Correspondence and requests for materials should be addressed to F.V.P. (email: fi[email protected]). NATURE COMMUNICATIONS | 4:1506 | DOI: 10.1038/ncomms2501 | www.nature.com/naturecommunications 1 & 2013 Macmillan Publishers Limited. All rights reserved. -

Increased S100B Blood Levels in Unmedicated and Treated
Molecular Psychiatry (2001) 6, 445–449 2001 Nature Publishing Group All rights reserved 1359-4184/01 $15.00 www.nature.com/mp ORIGINAL RESEARCH ARTICLE Increased S100B blood levels in unmedicated and treated schizophrenic patients are correlated with negative symptomatology M Rothermundt1, U Missler2, V Arolt1, M Peters1, J Leadbeater3, M Wiesmann2, S Rudolf4, KP Wandinger5 and H Kirchner4 1Department of Psychiatry, University of Muenster School of Medicine, Albert-Schweitzer-Str 11, D-48129 Muenster, Germany; 2Department of Neuroradiology, Medical University of Luebeck, Ratzeburger Allee 160, D-23538 Luebeck, Germany; 3Psychiatric Hospital, Friedrich-Ebert-Str, D-23774 Heiligenhafen, Germany; 4Institute of Immunology and Transfusion Medicine, Medical University of Luebeck, Ratzeburger Allee 160, D-23538 Luebeck, Germany; 5Department of Neurology, Charite Campus Mitte, NWFZ 2680, R 04 023, Schumannstr 20/21, D-10117 Berlin, Germany Keywords: nerve tissue protein S100; schizophrenia; anti- The term S100 comprises a heterogeneous family of psychotic agents; negative symptomatology; psychiatric acidic calcium-binding proteins of which the two pro- status teins S100A1 and S100B are considered to be the most S100B, a calcium-binding protein produced by astroglial relevant members regarding neurological disease.3 cells, is a marker of astroglial cellular integrity. It has S100B predominates in the brain. S100A1 and S100B been shown to be increased in acute brain damage and form dimeric proteins with a molecular weight of 21 neurodegeneration. A recent study showed increased kDA, which have previously been named S100a S100B levels in medicated acutely psychotic patients (S100A1–S100B), S100b (S100B–S100B), and S100a0 with schizophrenia. The study presented here included (S100A–S100A).4 S100B is synthesized mainly by 26 drug-free patients with acute schizophrenia and 26 astrocytes and evolves paracrine and autocrine effects matched healthy controls. -

Physiological and Pathophysiological Regulation of the Ryanodine Receptor in Skeletal Muscle
Physiological and pathophysiological regulation of the ryanodine receptor in skeletal muscle Alisa Umanskaya Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2015 © 2015 Alisa Umanskaya All rights reserved Abstract Physiological and pathophysiological regulation of ryanodine receptor in skeletal muscle Alisa Umanskaya Ryanodine receptor calcium release channels are essential for skeletal muscle contraction, as they mediate the release of calcium ions from intracellular stores into the cytosol. The data presented in this dissertation demonstrate the evolutionarily conserved mechanisms of skeletal muscle ryanodine receptor regulation in the physiological and pathophysiological states. Adrenergic stimulation causes increased skeletal muscle force, however, despite the well- established role of this physiological response, the molecular mechanism is not known. Here we present a mechanism whereby phosphorylation of a single amino acid on the ryanodine receptor is a key signal in the physiological stress-induced inotropic response in mouse skeletal muscle. Therefore acute post-translational modifications of ryanodine receptor channels are important for healthy muscle contraction. Conversely, chronic stress-induced post-translational modifications result in poorly functioning murine ryanodine receptor channels that contribute to skeletal muscle dysfunction in age- dependent skeletal muscle weakness and Muscular Dystrophies. Finally, we present data that demonstrates striking evolutionary conservation in ryanodine receptor regulation in the physiological and pathophysiological states between mice and C. elegans. This work has broad implications for understanding the underlying mechanisms of skeletal muscle contraction and important disorders that affect human health. Furthermore, this works presents ryanodine receptor channels as a viable therapeutic target for age-related skeletal muscle weakness, Muscular Dystrophies, and also implicates C. -

Spatial Distribution of Leading Pacemaker Sites in the Normal, Intact Rat Sinoa
Supplementary Material Supplementary Figure 1: Spatial distribution of leading pacemaker sites in the normal, intact rat sinoatrial 5 nodes (SAN) plotted along a normalized y-axis between the superior vena cava (SVC) and inferior vena 6 cava (IVC) and a scaled x-axis in millimeters (n = 8). Colors correspond to treatment condition (black: 7 baseline, blue: 100 µM Acetylcholine (ACh), red: 500 nM Isoproterenol (ISO)). 1 Supplementary Figure 2: Spatial distribution of leading pacemaker sites before and after surgical 3 separation of the rat SAN (n = 5). Top: Intact SAN preparations with leading pacemaker sites plotted during 4 baseline conditions. Bottom: Surgically cut SAN preparations with leading pacemaker sites plotted during 5 baseline conditions (black) and exposure to pharmacological stimulation (blue: 100 µM ACh, red: 500 nM 6 ISO). 2 a &DUGLDFIoQChDQQHOV .FQM FOXVWHU &DFQDG &DFQDK *MD &DFQJ .FQLS .FQG .FQK .FQM &DFQDF &DFQE .FQM í $WSD .FQD .FQM í .FQN &DVT 5\U .FQM &DFQJ &DFQDG ,WSU 6FQD &DFQDG .FQQ &DFQDJ &DFQDG .FQD .FQT 6FQD 3OQ 6FQD +FQ *MD ,WSU 6FQE +FQ *MG .FQN .FQQ .FQN .FQD .FQE .FQQ +FQ &DFQDD &DFQE &DOP .FQM .FQD .FQN .FQG .FQN &DOP 6FQD .FQD 6FQE 6FQD 6FQD ,WSU +FQ 6FQD 5\U 6FQD 6FQE 6FQD .FQQ .FQH 6FQD &DFQE 6FQE .FQM FOXVWHU V6$1 L6$1 5$ /$ 3 b &DUGLDFReFHSWRUV $GUDF FOXVWHU $GUDD &DY &KUQE &KUP &KJD 0\O 3GHG &KUQD $GUE $GUDG &KUQE 5JV í 9LS $GUDE 7SP í 5JV 7QQF 3GHE 0\K $GUE *QDL $QN $GUDD $QN $QN &KUP $GUDE $NDS $WSE 5DPS &KUP 0\O &KUQD 6UF &KUQH $GUE &KUQD FOXVWHU V6$1 L6$1 5$ /$ 4 c 1HXURQDOPURWHLQV -

Preclinical Model Systems of Ryanodine Receptor 1-Related Myopathies and Malignant Hyperthermia
Lawal et al. Orphanet Journal of Rare Diseases (2020) 15:113 https://doi.org/10.1186/s13023-020-01384-x REVIEW Open Access Preclinical model systems of ryanodine receptor 1-related myopathies and malignant hyperthermia: a comprehensive scoping review of works published 1990– 2019 Tokunbor A. Lawal1, Emily S. Wires2, Nancy L. Terry3, James J. Dowling4 and Joshua J. Todd1* Abstract Background: Pathogenic variations in the gene encoding the skeletal muscle ryanodine receptor (RyR1) are associated with malignant hyperthermia (MH) susceptibility, a life-threatening hypermetabolic condition and RYR1- related myopathies (RYR1-RM), a spectrum of rare neuromuscular disorders. In RYR1-RM, intracellular calcium dysregulation, post-translational modifications, and decreased protein expression lead to a heterogenous clinical presentation including proximal muscle weakness, contractures, scoliosis, respiratory insufficiency, and ophthalmoplegia. Preclinical model systems of RYR1-RM and MH have been developed to better understand underlying pathomechanisms and test potential therapeutics. Methods: We conducted a comprehensive scoping review of scientific literature pertaining to RYR1-RM and MH preclinical model systems in accordance with the PRISMA Scoping Reviews Checklist and the framework proposed by Arksey and O’Malley. Two major electronic databases (PubMed and EMBASE) were searched without language restriction for articles and abstracts published between January 1, 1990 and July 3, 2019. Results: Our search yielded 5049 publications from which 262 were included in this review. A majority of variants tested in RYR1 preclinical models were localized to established MH/central core disease (MH/CCD) hot spots. A total of 250 unique RYR1 variations were reported in human/rodent/porcine models with 95% being missense substitutions.