The Pennsylvania State University
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

International Standard Nace Mr0175/ Iso 15156-1
INTERNATIONAL NACE STANDARD MR0175/ ISO 15156-1 Item No. 21306 First edition Petroleum and natural gas industries— Materials for use in H2S-containing Environments in oil and gas production— Part 1: General principles for selection of cracking-resistant materials Reference number NACE MR0175/ISO 15156-1:2001(E) ISBN 1-57590-1765 © NACE International/ISO 2001 NACE MR0175/ISO 15156-1:2001(E) © NACE International/ISO 2001 These materials are subject to copyright claims of ISO, ANSI, and NACE. No part of this publication may be reproduced in any form, including an electronic retrieval system, without the prior written permission of NACE. All requests pertaining to the NACE MR0175/ISO 15156 standard should be submitted to NACE. International Organization for Standardization (ISO) Case postale 56 • CH-1211 Geneva 20 Tel. + 41 22 749 01 11 Fax + 41 22 749 09 47 Web www.iso.ch American National Standards Institute (ANSI) 25 W. 43rd St., Fourth Floor New York, NY 10036 Tel. +1 212-642-4900 Fax +1 212-398-0023 Web www.ansi.org NACE International 1440 South Creek Dr. Houston, TX 77084-4906 Tel. +1 281-228-6200 Fax +1 281-228-6300 E-mail [email protected] Web: www.nace.org Printed in the U.S.A. by NACE ii NACE International/ISO NACE MR0175/ISO 15156-1:2001(E) Contents 1 Scope ......................................................................................................................... 1 2 Normative references................................................................................................. 2 3 Terms and definitions................................................................................................ -

(12) United States Patent (10) Patent No.: US 6,306,544 B1 Frysz Et Al
USOO630654.4B1 (12) United States Patent (10) Patent No.: US 6,306,544 B1 Frysz et al. (45) Date of Patent: Oct. 23, 2001 (54) COBALT-BASED ALLOYS AS POSITIVE 5,051,233 9/1991 Horn et al. ............................. 420/50 ELECTRODE CURRENT COLLECTORS IN 5,053,297 10/1991 Yamahira et al. ................... 429/194 NONAQUEOUSELECTROCHEMICAL 5,114,810 * 5/1992 Frysz et al. ...... 429/194 CELLS 5,147,739 9/1992 Beard ....... ... 429/194 5,187,033 2/1993 Koshiba ....... ... 429/194 (75) Inventors: Christine A. Frysz, East Amherst; 2. : E. Tateishi et al. ...................... 428/548 2 : Y-2 /1995 Idota .................................... 429/194 Sally Ann Smesko, North Tonawanda; 5,578,396 * 11/1996 Fauteux et al. .. 429/209 Peter A. Kreidler, Oakfield; W. 5,643,690 * 7/1997 Tateishi et al. .. ... 429/34 Richard Brown, Clarence Center; 5,733,680 * 3/1998 Hong ............ ... 429/101 Esther S. Takeuchi, East Amherst, all 5,783,333 * 7/1998 Mayer .............. ... 429/223 of NY (US) 5,795,679 * 8/1998 Kawakami et al. 429/218 5.962,160 * 5/1999 Oyama et al. ....................... 429/104 (73) Assignee: Wilson Greatbatch Ltd., Clarence, NY 6,136,477 10/1998 Gan et al. ............................ 429/307 (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this WO9833224 7/1998 (WO). patent is extended or adjusted under 35 U.S.C. 154(b) by 0 days. OTHER PUBLICATIONS Standard Specification for Wrought Cobalt-Nickel-Chromi (21) Appl. No.: 09/257,795 um-Molybdenum-Tungsten-Iron Alloy For Surgical Implant Applications; pp. 102-104; Designation F563-88. -

General Disclaimer One Or More of the Following Statements May Affect
General Disclaimer One or more of the Following Statements may affect this Document This document has been reproduced from the best copy furnished by the organizational source. It is being released in the interest of making available as much information as possible. This document may contain data, which exceeds the sheet parameters. It was furnished in this condition by the organizational source and is the best copy available. This document may contain tone-on-tone or color graphs, charts and/or pictures, which have been reproduced in black and white. This document is paginated as submitted by the original source. Portions of this document are not fully legible due to the historical nature of some of the material. However, it is the best reproduction available from the original submission. Produced by the NASA Center for Aerospace Information (CASI) ,-M (NASA-CR-140957) THE RESTSTANCB OF N75-12115 a ; SELECTED HIGH STRENGTH ALLOYS TO E&BRITTLEMENT BY A HYDROGEN ENVZhONMENT (Worth Carolina Stake Univ.) 13 p HC Unclas ^ y $3.25 CSCL 11F G3/26 02808 k' 1 AEG r 3 0' E ' E . Y xr - THE RESISTANCE OF SLLECTED HIGH STRENGTH r. ALLOTS TO EMBRITTLEMENT BY A HYDROGEN ENVIRONMENT S i _. Ray B. Benson, Jr. P North ,Carolina State University Raleigh, North Carolina 4 } 3l ABSTRACT Selected high strength iron base and cobalt base alloys with yield strengths in the range of 1233 to 2129'N1Nm' 2 (179-309 KSI)— were resistant to degradation of mechanical properties in a one ( p^{{^j` atmosphere hydrogen environment at ambient temperature. These alloys were strengthened initially by cold working which produced strain induced e'-lisp martensite and fcc mechanical twins in an 1 ; fcc matrix. -

Rolled Products Brochure
Precision Quality Experience Precision The Advantage of Arnold Magnetic Technologies Arnold Magnetics’ Rolled Products Division was established in 1950 to manufacture foil and precision strip alloys for our in-house production of tape-wound magnetic cores. The need for precise thickness and process control required us to develop specialized processes that were capable of achieving necessary characteristics in a variety of alloys. Our experience with these techniques and capabilities earned us a reputation as a premier custom processor of a wide spectrum of alloys. Arnold Magnetics’ foil and precision strip products are preferred by many companies in aerospace, medical electronics, telecommunications, instruments, sensors, electrical, and many metalworking industries. To ensure continued conformance with the quality demanded by today’s competitive environment, Arnold Magnetics has invested in personnel, training, equipment and quality control programs to meet our customers’ requirements. “We judge our performance These processes produce components for such critical applications as springs, strain gauges, shielding, gaskets, brazing, magnetic and on how well we satisfy our electromagnetic sensors, honeycomb structures, implant devices, transformers and motor laminates, and many other precision customers’ needs.” applications. Capabilities ■ Precision thin-gauge alloy foil available in: sheet, strip and continuous coils. Available widths from 16.5" to a minimum of 0.035" (0.9 mm). Minimum thickness of 0.0005" (0.0125 mm) for 4 to 16.5" in width and 0.000085" (2.16 micron) for 4" widths and less. ■ Arnold offers wire, rod and bar stock for selected magnetic alloys such as the ductile ARNOKROME™ III alloy developed by Arnold based on the iron-chrome-cobalt alloy system. -
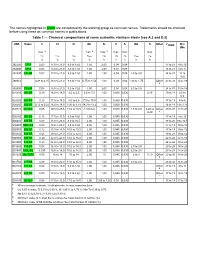
The Names Highlighted in Green Are Considered by the Working Group As Common Names
The names highlighted in green are considered by the working group as common names. Trademarks should be checked before using these as common names in publications. Table 1 — Chemical compositions of some austenitic stainless steels (see A.2 and D.3) UNS Name C Cr Ni Mn Si P S Mo N Other FPREN Ni + 2Mo max. a max. a max. a max. max. max. wC wCr wNi wMn wSi wP wS wMo wN % % % % % % % % % J92500 CF-3 0,03 17,0 to 21,0 8,0 to 12,0 1,50 2,00 0,04 0,04 17 to 21 8 to 12 J92600 CF-8 0,08 18,0 to 21,0 8,0 to 11,0 1,50 2,00 0,04 0,04 18 to 21 8 to 11 J92800 CF-3M 0,03 17,0 to 21,0 9,0 to 13,0 1,50 1,50 0,04 0,04 2,0 to 3,0 24 to 31 11 to 16,5 J92843 0,28 to 0,35 18,0 to 21,0 8,0 to 11,0 0,75 to 1,50 1,00 0,04 0,04 1,00 to 1,75 Other 23 to 30 14 to 19 b J92900 CF-8M 0,08 18,0 to 21,0 9,0 to 12,0 1,50 2,00 0,04 0,04 2,0 to 3,0 24 to 31 13 to 18 S20100 201 SS 0,15 16,0 to 18,0 3,5 to 5,5 5,5 to 7,5 1,00 0,060 0,030 0,25 20 to 22 3,5 to 5,5 S20200 202 SS 0,15 17,0 to 19,0 4,0 to 6,0 7,5 to 10,0 1,00 0,060 0,030 17 to 19 4 to 6 S20500 205 SS 0,12 to 0,25 16,0 to 18,0 1,00 to 1,75 14,0 to 15,5 1,00 0,060 0,030 16 to 18 1 to 1,75 S20910 22-13-5 0,06 20,5 to 23,5 11,5 to 13,5 4,0 to 6,0 1,00 0,040 0,030 1,5 to 3,0 0,20 to Other 29 to 38 14 to 20 0,40 c S30200 302 SS 0,15 17,0 to 19,0 8,0 to 10,0 2,00 1,00 0,045 0,030 17 to 19 8 to 10 S30400 304 SS 0,08 18,0 to 20,0 8,0 to 10,5 2,00 1,00 0,045 0,030 18 to 20 8 to 10,5 S30403 304L SS 0,03 18,0 to 20,0 8,0 to 12,0 2,00 1,00 0,045 0,030 17 to 19 10 to 13 S30500 305 SS 0,12 17,0 to 19,0 10,0 to 13,0 2,00 1,00 -
![Measurement of Long Lived Radioactive Impurities Retained in the Disposable Cassettes on the Tracerlab MX System During the Production of [18F]FDG](https://docslib.b-cdn.net/cover/9754/measurement-of-long-lived-radioactive-impurities-retained-in-the-disposable-cassettes-on-the-tracerlab-mx-system-during-the-production-of-18f-fdg-4889754.webp)
Measurement of Long Lived Radioactive Impurities Retained in the Disposable Cassettes on the Tracerlab MX System During the Production of [18F]FDG
Applied Radiation and Isotopes ] (]]]]) ]]]–]]] Contents lists available at ScienceDirect Applied Radiation and Isotopes journal homepage: www.elsevier.com/locate/apradiso Measurement of long lived radioactive impurities retained in the disposable cassettes on the Tracerlab MX system during the production of [18F]FDG D. Ferguson n, P. Orr, J. Gillanders, G. Corrigan, C. Marshall Regional Medical Physics Service, Belfast Health and Social Care Trust, Belfast, Northern Ireland article info abstract Article history: Using a High- Purity Germanium gamma-ray spectrometer, a number of radioisotopes have been Received 15 December 2010 identified within Tracerlab MX radiochemistry system cassettes used to synthesise [18F]FDG. Twenty Received in revised form radiochemistry cassettes were measured and the average total activity of each radioisotope was 20 May 2011 determined. Using these values and decay correction, the minimum time the cassettes should be left in Accepted 22 May 2011 a decay store before the specific activity falls below 0.4B q/g, the limit for disposal alongside Clinical Waste was found to be 24 months. Keywords: & 2011 Elsevier Ltd. All rights reserved. Cyclotron FDG Waste disposal Radioactive waste 1. Introduction Since 18FÀ is produced by the 18O(p,n)18F reaction, it is neces- sary to remove the 18F-ion from its aqueous environment. On the 18F is the most widely used radionuclide in PET and is GE Tracerlab MX system, which utilises disposable cassettes commonly produced by bombarding 18O enriched water with (supplied by Rotem Industries, Ltd), this is performed using a QMA accelerated protons. It is well established that long lived radio- Sep-Pak column (supplied as a part of ABX reagent kit, Biomedizi- nuclide impurities are generated using this method as a result of nische Forschungsreagenzien GmbH). -
Long-Lived Contaminants in Short-Lived Cyclotron-Produced Radiopharmaceuticals with Visual Robfit
Long-lived contaminants in short-lived cyclotron-produced radiopharmaceuticals with Visual RobFit Robert Metzger1,a and George Lasche2 1Radiation Safety Engineering, Inc, 3245 North Washington Street, Chandler, AZ, USA 2Snakedance Scientific, LLC, 13509 Quaking Aspen Place NE, Albuquerque, NM, USA Abstract. Oxygenated target waters of cyclotron targets contain long-lived contaminants due to (p,n) reactions in the Havar target window that are spalled into the target water. These contaminants must be scrubbed out of the water as part of the drug synthesis process. Currently the USP requires that the final drug product be 99.5% pure, so the total activity of the long-lived contaminants can be no more than 0.5% of the final radiopharmaceutical product. A method of determining these long-lived contaminants with whole-spectrum non-linear shape fitting has been developed using high-resolution gamma spectroscopy. Results of whole-spectrum shape fitting are compared to results from standard software that uses results of an initial search of individual peaks. It is shown that the usual problems due to interference corrections of individually-fitted peaks are not encountered with whole-spectrum shape fitting, with improved overall analytic results. 1 Introduction lived contaminants that are evident with gamma spectroscopy are 55Co, 56Co, 57Co, 58Co , 51Cr, 52Mn, 54Mn, For positron-emission tomography (PET) short-lived 57Ni, 183Re, 95Tc, 95mTc, and 96Tc. In common practice the radionuclides that emit no gamma-rays but only positrons activity concentrations -

Physical and Mechanical Properties of Co Based Thin Films for Window
Physical and Mechanical Properties of Co Based Thin Films for Window Targets in Accelerators Thesis submitted in partial fulfillment of the requirements for the degree of “DOCTOR OF PHILOSOPHY” by Shlomo Haroush 01/12/2016 Submitted to the Senate of Ben-Gurion University of the Negev Beer-Sheva Physical and Mechanical Properties of Co Based Thin Films for Window Targets in Accelerators Thesis submitted in partial fulfillment of the requirements for the degree of “DOCTOR OF PHILOSOPHY” by Shlomo Haroush May 2017 Submitted to the Senate of Ben-Gurion University of the Negev Beer-Sheva Physical and Mechanical Properties of Co Based Thin Films for Window Targets in Accelerators Thesis submitted in partial fulfillment of the requirements for the degree of “DOCTOR OF PHILOSOPHY” by Shlomo Haroush Submitted to the Senate of Ben-Gurion University of the Negev Approved by the advisors Prof. Roni Z. Shneck: ________________________ 01/05/2017 Prof. Yaniv Gelbstein: ________________________ 01/05/2017 Approved by the Dean of the Kreitman School of Advanced Graduate Studies: Prof. Michal Shapira: ____________________________ May 2017 Beer-Sheva This work was carried out under the supervision of Prof. Roni Z. Shneck Prof. Yaniv Gelbstein In the Department of Materials Engineering Faculty of Engineering Sciences Research Student's Affidavit when Submitting the Doctoral Thesis for Judgment I, Shlomo Haroush, whose signature appears below, hereby declare that: I have written this Thesis by myself, except for the help and guidance offered by my Thesis Advisors. The scientific materials included in this Thesis are products of my own research, culled from the period during which I was a research student. -

CONSIDERATIONS, MEASUREMENTS, and LOGISTICS ASSOCIATED with LOW-ENERGY CYCLOTRON DECOMMISSIONING Sunderland J.J., Erdahl C.E., Bender B.R.,Sensoy L., Watkins G.L
CONSIDERATIONS, MEASUREMENTS, AND LOGISTICS ASSOCIATED WITH LOW-ENERGY CYCLOTRON DECOMMISSIONING Sunderland J.J., Erdahl C.E., Bender B.R.,Sensoy L., Watkins G.L. Positron Emission Tomography Imaging Center, University of Iowa, Iowa City, IA, USA Corresponding author email address: [email protected] pCi/gm ± Half-Life Likely Production Channel Introduction: There are currently approximately 150 active PET cyclotrons in the US. Ce-141 0.043 0.019 32 days (n,γ) on natural cerium in concrete There are likely at least 3X that number, total, in the world. Despite the fact that Co-60 1.835 0.042 5.27 years (n,γ) on natural cobalt in concrete the first wave of PET cyclotrons is in the decommissioning stage, there is still Cs-134 0.128 0.023 2.06 years (n, γ) on natural cesium in concrete little information in the literature on the science, health physics, and regulatory Eu-152 2.23 0.086 13.3 years (n, γ) on natural europium in concrete framework surrounding the process. Here we present quantitative radioactive Eu-154 0.264 0.047 8.8 years (n, ) on natural europium in concrete waste measurements associated with the University of Iowa’s cyclotron γ decommissioning. K-40 8.16 0.386 1.3e9 years Naturally Occurring La-140 0.528 0.269 40.3 hours (n, γ) on natural lanthanum in concrete Mn-54 0.436 0.036 312 days (n,p) on natural iron in concrete Material and methods: Na-24 211.4 2.08 15 hours (n, γ) on natural sodium in concrete In the summer of 2011, the University of Iowa’s 20-year-old 17 MeV And (n, α) on natural aluminum Scandatronix cyclotron underwent decommissioning. -

Xerox University Microfilms
INFORMATION TO USERS This material was produced from a microfilm copy of the original document. While the most advanced technological means to photograph and reproduce this document have been used, the quality is heavily dependent upon the quality of the original submitted. The following explanation of techniques is provided to help you understand markings or patterns which may appear on this reproduction. 1.The sign or "target" for pages apparently lacking from the document photographed is "Missing Page(s)". If it was possible to obtain the missing page(s) or section, they are spliced into the film along with adjacent pages. This may have necessitated cutting thru an image and duplicating adjacent pages to insure you complete continuity. 2. When an image on the film is obliterated with a large round black mark, it is an indication that the photographer suspected that the copy may have moved during exposure and thus cause a blurred image. You will find a good image of the page in the adjacent frame. 3. When a map, drawing or chart, etc., was part of the material being photographed the photographer followed a definite method in "sectioning" the material. It is customary to begin photoing at the upper left hand corner of a large sheet and to continue photoing from left to right in equal sections with a small overlap. If necessary, sectioning is continued again — beginning below the first row and continuing on until complete. 4. The majority of users indicate that the textual content is of greatest value, however, a somewhat higher quality reproduction could be made from "photographs" if essential to the understanding of the dissertation. -

Metal Strip Brochure
INNOVATIVE & ADVANCED METALLURGICAL TECHNOLOGY • Strip • Strip • Shaped Wire • Foil • Near Net Shaped Components • Ultra-thin Foil • Thermal Management Materials WALLINGFORD METAL STRIP METAL STRIP PRODUCTS NICKEL STRIP • Offering more alloy variations through powder High-Purity Dispersed Phase metallurgy than any other producer. AME 270 - 899A AME 201 - 899E • Tightly controlled chemistries result in high- AME 200 - 899L AME 233 - 899D purity strip with extremely low impurity levels AME 201 - 899M AME 205 - 899G & surface oxides. • Featuring tight tolerance, high quality surface ALLOY AMETEK SMP INDUSTRY finish & slit edges. SYSTEM GRADE EQUIVALENT 936 Invar® • Smaller lot sizes at competitive prices. 942 Alloy 42 946 Alloy 46 STANDARD CAPABILITIES Nickel Iron Strip 948 Alloy 48 • Thickness: 952 Alloy 52 0.002” - 0.090” ± 5% Others Available • Width: Fe-Ni-Co Strip 515 Sealvar® F-15 Alloy Kovar®, Rodar® 0.062” - 14.00” (and up to 22.00” in some 891 3% up to 14% W available cases, depending on gauge & temper) - +/- .005” Nickel 886 3% up to 14% W available • Coil Size: Tungsten Strip Others Available Up to 200 lbs. PIW, or 6000 lbs, with no welds. 0.062” - 14.00” (and up to 22.00” in Ductile Cobalt Cobalt Strip 580, 591, 595 - some cases, depending on gauge & temper) - High Purity Cobalt 599 +/- .005”. Standard arbor dia. of 3”, 6”, 8”, 12”, 16” Co, Ni, NiFe offered. Oscillate-wound coils up to 1,000 lbs. Weld Wire - Others available • Temper Range: Cu-Ni-Sn 377 Pfinodal® C72900 Annealed DDQ to Full Hard, incl. ¼ H, ½ H, ¾ H. Alloy Strip 388 Spinodal C72650 80/20, 70/30 Ni-Cr - and 65/35 High-Purity Fe 900B HP Iron ABOUT WALLINGFORD Putting metal strip technology to work for you.. -

Cyclotron Produced Radionuclides: Principles and Practice and Principles Radionuclides: Produced Cyclotron Cyclotron Produced Radionuclides: Principles and Practice
232 pages 13.76 mm technical reportS series no. This book provides a comprehensive treatment of cyclotrons, with a special emphasis on pro- duction of radionuclides. Individual sections are devoted to accelerator technology, theoreti- cal aspects of nuclear reactions, the technol- ogy behind targetry, techniques for preparation of targets, irradiation of targets under high 465 beam currents, target processing and target recovery. This book will appeal to scientists and Technical Reports SeriEs No. 465 technologists interested in translating cyclo- tron technology into practice, as well as post- graduate students in this field. Cyclotron Produced Radionuclides: Principles and Practice Cyclotron Produced Radionuclides: Principles and Practice INTERNATIONAL ATOMIC ENERGY AGENCY VIENNA ISBN 978–92–0–100208–2 ISSN 0074–1914 D465_covI-IV.indd 1 2008-10-21 13:52:56 CYCLOTRON PRODUCED RADIONUCLIDES: PRINCIPLES AND PRACTICE The following States are Members of the International Atomic Energy Agency: AFGHANISTAN GUATEMALA PAKISTAN ALBANIA HAITI PALAU ALGERIA HOLY SEE PANAMA ANGOLA HONDURAS PARAGUAY ARGENTINA HUNGARY PERU ARMENIA ICELAND PHILIPPINES AUSTRALIA INDIA POLAND AUSTRIA INDONESIA PORTUGAL AZERBAIJAN IRAN, ISLAMIC REPUBLIC OF QATAR BANGLADESH IRAQ REPUBLIC OF MOLDOVA BELARUS IRELAND ROMANIA BELGIUM ISRAEL RUSSIAN FEDERATION BELIZE ITALY SAUDI ARABIA BENIN JAMAICA SENEGAL BOLIVIA JAPAN SERBIA BOSNIA AND HERZEGOVINA JORDAN BOTSWANA KAZAKHSTAN SEYCHELLES BRAZIL KENYA SIERRA LEONE BULGARIA KOREA, REPUBLIC OF SINGAPORE BURKINA FASO KUWAIT SLOVAKIA