Geochemistry of El-Salam Canal and the Adjacent Groundwater in North
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Econbc Feasibility Study I 220
VTT TECHNOLOGY NOL CH OG E Y T • • R E E C S N E E A EcoNBC feasibility study I 220 R C 220 C Transforming New Borg El Arab into an EcoCity S H • S H N I G O I H S L New Borg El Arab was inaugurated in 1988 and is seen as the I I V G • H S natural extension of Alexandria, as well as one of the most T important industrial areas in Egypt. Transforming New Borg El Arab EcoNBC feasibility study City into an EcoCity was one of the main drivers for EcoNBC project (EcoCity Capacity Building in New Borg El Arab City). Carrying out a Feasibility Study to explore more in detail the viability of the idea was one of the first steps to be taken towards that transformation. In this case, it is the result of the joint effort of a team of Finnish and Egyptian experts through a series of structured workshops that took place both in Finland and Egypt, and a number of focused discussions that involved also key stakeholders. An insight into the different economic sectors has been taken and a vision of how the main issues of concern selected, namely Energy, Water and Water, should be approached from each of those sectors has been defined, of course with a special emphasis on the sustainability of the Ecosystem. This has allowed the expert team responsible for this Feasibility Study to propose practical solutions in the form of scenarios. In the case of some sectors where reliable data was available for the calculations, these scenarios have been developed down to their associated impacts. -

Shereif Hassan Mahmoud
Hydroclimate Changes to Arid regions subjected to Impact of climate change, human activities, and Large-scale climate patterns by Shereif Hassan Mahmoud A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Water Resources Engineering Department of Civil and Environmental Engineering University of Alberta © Shereif Hassan Mahmoud, 2020 Abstract In recent years, many regions worldwide have suffered from natural hazards related to the impact of human activities and climate change, such as floods and droughts, sea level rise, extreme weather events and an accelerated hydrological cycle. In Africa, the driest continent on Earth, climate change has led to more frequent occurrences of droughts of greater severity. Beside climate change, human activities have also incurred negative environmental impact which in turn has likely affected the climate at a wide range of temporal-spatial scales worldwide. For example, in the Middle East, floods of greater magnitude have been occurring more frequently in recent decades, which could be attributed partly to rapid urbanization or the effect of climate change, or both. In the Nile River basin (NRB), recurring droughts and increasing population have led to rising tension between competing users for water. Therefore, to develop more effective mitigation strategies against the potential impact of climate change, there is an urgent need to better understand changes to the hydrologic cycle of arid regions and linkage to regional climate change. The objectives of this dissertation are: 1) To investigate the potential implications of urbanization and climate change to the flood risk of Egypt and Saudi Arabia of arid climate in the Middle East. -

País Região Cidade Nome De Hotel Morada Código Postal Algeria
País Região Cidade Nome de Hotel Morada Código Postal Algeria Adrar Timimoun Gourara Hotel Timimoun, Algeria Algeria Algiers Aïn Benian Hotel Hammamet Ain Benian RN Nº 11 Grand Rocher Cap Caxine , 16061, Aïn Benian, Algeria Algeria Algiers Aïn Benian Hôtel Hammamet Alger Route nationale n°11, Grand Rocher, Ain Benian 16061, Algeria 16061 Algeria Algiers Alger Centre Safir Alger 2 Rue Assellah Hocine, Alger Centre 16000 16000 Algeria Algiers Alger Centre Samir Hotel 74 Rue Didouche Mourad, Alger Ctre, Algeria Algeria Algiers Alger Centre Albert Premier 5 Pasteur Ave, Alger Centre 16000 16000 Algeria Algiers Alger Centre Hotel Suisse 06 rue Lieutenant Salah Boulhart, Rue Mohamed TOUILEB, Alger 16000, Algeria 16000 Algeria Algiers Alger Centre Hotel Aurassi Hotel El-Aurassi, 1 Ave du Docteur Frantz Fanon, Alger Centre, Algeria Algeria Algiers Alger Centre ABC Hotel 18, Rue Abdelkader Remini Ex Dujonchay, Alger Centre 16000, Algeria 16000 Algeria Algiers Alger Centre Space Telemly Hotel 01 Alger, Avenue YAHIA FERRADI, Alger Ctre, Algeria Algeria Algiers Alger Centre Hôtel ST 04, Rue MIKIDECHE MOULOUD ( Ex semar pierre ), 4, Alger Ctre 16000, Algeria 16000 Algeria Algiers Alger Centre Dar El Ikram 24 Rue Nezzar Kbaili Aissa, Alger Centre 16000, Algeria 16000 Algeria Algiers Alger Centre Hotel Oran Center 44 Rue Larbi Ben M'hidi, Alger Ctre, Algeria Algeria Algiers Alger Centre Es-Safir Hotel Rue Asselah Hocine, Alger Ctre, Algeria Algeria Algiers Alger Centre Dar El Ikram 22 Rue Hocine BELADJEL, Algiers, Algeria Algeria Algiers Alger Centre -

SHERIF M ABDELMOHSEN Phd (Georgia Tech), Msc, Bsc (Honors)
RESUME JUL 2017 SHERIF M ABDELMOHSEN PhD (Georgia Tech), MSc, BSc (Honors) ASSOCIATE PROFESSOR OF DIGITAL MEDIA & DESIGN COMPUTING IN ARCHITECTURE Department of Architecture, The American University in Cairo (AUC), Cairo, Egypt CO-FOUNDER, UDAAR Email: [email protected] URL: https://aucegypt.academia.edu/SHERIFMORADABDELMOHSEN http://www.aucegypt.edu/fac/Profiles/Pages/SherifAbdelmohsen.aspx EDUCATION PhD in Architecture (2011) School of Architecture, Georgia Institute of Technology, Atlanta, GA, USA PhD Thesis: An Ethnographically Informed Analysis of Design Intent Communication in BIM-Enabled Architectural Practice Recipient of Faculty Award of Merit for Best Doctoral Presentation (2010/2011) MSc in Architecture (2004) Department of Architecture, Ain Shams University, Cairo, Egypt MSc Thesis: Towards a Conceptual Framework for Implementing Intelligent Mixed Reality in Architectural Education BSc in Architecture (2000) (Honors) Department of Architecture, Ain Shams University, Cairo, Egypt BSc Graduation Project: Touristic Cultural Complex at Cairo Northern Wall POSITION Associate Professor (01/2017 – Present) Associate Professor of Digital Media and Design Computing in Architecture Department of Architecture, School of Sciences and Engineering (SSE), The American University in Cairo (AUC), Egypt EMPLOYMENT April 2017 – Present UDAAR for Engineering Consultancy and Technology Solutions Founder, Director of Research and Development August 2014 – December 2016 Department of Architecture, American University in Cairo (AUC), Egypt -

Readiness for Ecocities in Egypt. Insights Into the Current State of Ecocity Systems, Technologies and Concepts
NOL CH OG E Y T • • R E E C S N E E A I 161 R C C S H • S H N I G O I H S L I I V G • H S T Readiness for EcoCities in Egypt Insights into the current state of EcoCity systems, technologies and concepts Åsa Hedman | Carmen Antuña Rozado | Ola Balbaa | Yehia ElMahgary | Ali El-Nashar | Ahmed ElShazly | Mona GamalEldin | Ahmad Hamza | Jutta Jantunen | Ali Kamel | Abdelazim Negm | Heba Saeed | Boshra Salem | Mohamed Shahin | Ahmed Tawfik| Pekka Tuominen | Walaa Youssef | Ahmed Yousry VTT TECHNOLOGY 161 Readiness for EcoCities in Egypt Insights into the current state of EcoCity systems, technologies and concepts Åsa Hedman, Carmen Antuña Rozado, Jutta Jantunen & Pekka Tuominen VTT Technical Research Centre of Finland Ola Balbaa, Yehia ElMahgary, Ahmed ElShazly, Mona GamalEldin, Ahmad Hamza, Ali Kamel, Abdelazim Negm, Heba Saeed & Ahmed Tawfik E-JUST Egypt-Japanese University of Science and Technology Ali El-Nashar, Boshra Salem, Mohamed Shahin & Walaa Youssef Alexandria University Ahmed Yousry Cairo University ISBN 978-951-38-8136-8 (URL: http://www.vtt.fi/publications/index.jsp) VTT Technology 161 ISSN-L 2242-1211 ISSN 2242-122X (Online) Copyright © VTT 2014 JULKAISIJA – UTGIVARE – PUBLISHER VTT PL 1000 (Tekniikantie 4 A, Espoo) 02044 VTT Puh. 020 722 111, faksi 020 722 7001 VTT PB 1000 (Teknikvägen 4 A, Esbo) FI-02044 VTT Tfn +358 20 722 111, telefax +358 20 722 7001 VTT Technical Research Centre of Finland P.O. Box 1000 (Tekniikantie 4 A, Espoo) FI-02044 VTT, Finland Tel. +358 20 722 111, fax +358 20 722 7001 Readiness for EcoCities in Egypt Insights into the current state of EcoCity systems, technologies and concepts Åsa Hedman, Carmen Antuña Rozado, Ola Balbaa, Yehia ElMahgary, Ali El-Nashar, Ahmed ElShazly, Mona GamalEldin, Ahmad Hamza, Jutta Jantunen, Ali Kamel, Abdelazim Negm, Heba Saeed, Boshra Salem, Mohamed Shahin, Ahmed Tawfik, Pekka Tuominen, Walaa Youssef & Ahmed Yousry. -

Unilever Mashreq for Manufacturing and Trading (S.A.E)
Current issue date: 23 June 2020 Original approval(s): Expiry date ISO14001: 26 June 2023 ISO 14001 - 17 July 2003 Expiry date ISO 9001: 26 June 2023 ISO 9001 - 27 June 2005 Expiry date OHSAS: 11 September 2021 OHSAS 18001 - 4 August 2005 Certificate identity number: 10275014 Certificate of Approval This is to certify that the Management System of: Unilever Mashreq for Manufacturing and Trading (S.A.E) Unilever Mashreq for Manufacturing, and Trading (S.A.E), Head Office, Smouha, Alexandria, Egypt has been approved by Lloyd's Register to the following standards: ISO 14001:2015, ISO 9001:2015, OHSAS 18001:2007 Approval number(s): ISO 14001 – 0045552, ISO 9001 – 0045553, OHSAS 18001 – 0045554 This certificate is valid only in association with the certificate schedule bearing the same number on which the locations applicable to this approval are listed. The scope of this approval is applicable to: Manufacturing and processing of foods, tea, personal care products, personal wash products and powder detergent. Luis Cunha Area Operations Manager - SAMEA Issued by: Lloyd's Register Egypt LLC for and on behalf of: Lloyd's Register Quality Assurance Limited Lloyd's Register Group Limited, its affiliates and subsidiaries, including Lloyd's Register Quality Assurance Limited (LRQA), and their respective officers, employees or agents are, individually and collectively, referred to in this clause as 'Lloyd's Register'. Lloyd's Register assumes no responsibility and shall not be liable to any person for any loss, damage or expense caused by reliance on the information or advice in this document or howsoever provided, unless that person has signed a contract with the relevant Lloyd's Register entity for the provision of this information or advice and in that case any responsibility or liability is exclusively on the terms and conditions set out in that contract. -

Stations and Monitoring Locations
National Water Research Center Research Stations and Monitoring Locations 2020 There are (26) research stations affiliated to seven institutes and units in addition to the Water Studies and Research Complex under the umbrella of the National Water Research Center (NWRC). Their research activities vary, as they support the NWRC research plan. 1. Carrying out marine measurements at the Egyptian coastal line. 2. Salt water desalination studies. 3. Fish farming, control of aquatic weeds, and production of biogas from aquatic weeds. 4. Reuse of drainage water for irrigation. 5. Experimenting sub-surface drainage systems. 6. Studying the crops water requirements and evaluating the modern irrigation systems. 7. Conducting experiments for artificial charging of groundwater. Under the umbrella of the NWRC, Its Institutes have monitoring locations besides the research stations cover the whole country as seen in the below geographical map.. 1 1- Water Management Research Institute The institute has 12 research stations, as seen in the below geographical map, cover Nile valley of Egypt with varied climate and soil conditions. In general, the purpose of the stations is experimenting the water requirements of different crops under different irrigation systems prevailing in the research regions. 1-1 El-Tal El-Kabeer station (East Delta region) Station location At Qantara and Hawis Al-Salihiya, before the city of El-Tal El- Kabeer. The area of station is 9 acres and mostly is sandy soils. Purpose of station • Studying the water requirements of different crops under the different irrigation systems prevailing in the region. 2 • Representing research experiments of dense sandy soils in the area. -

Alexmareotis-Khalil2
E. KHALIL: MAREOTIS’ WATERFRONT Waterfront Installations and Maritime Activities in the Mareotic Region Emad Khalil Introduction tangular body of water which merged to the east and south Lake Mareotis represents one of the most distinctive geo- with the Nile Delta Plain, and a narrow arm that extended morphic features in the north-west coastal region of Egypt. westwards parallel to the northern coast. However, dur- In antiquity, it was fed by means of a number of canals, ing the past two millennia Lake Mareotis has undergone which bifurcated off the Nile’s defunct Canopic Branch, dramatic changes which significantly affected its size and and flowed into the southern and eastern sides of the lake nature. Nonetheless, the western extremities of the lake re- (Fig. 1). Some of these canals were navigable, which ena- flect the original extant remains, and form an arm, known bled merchandise to be transported to and from the hinter- as the Mareotic Arm, that extends some 40 km west of land. By the Greco-Roman period the lake was also con- Alexandria and is 2-3 km wide and is separated from the nected to the sea through a navigable canal that debouched lake’s main body by causeways and shallows. It also con- at Alexandria (Strabo 17.1.7). Its connection to both the tains an island, Mareotis Island, which is 3.7 km long and Nile and the sea resulted in Mareotis becoming a vital con- about 680 m at its widest point (see both Blue and Hopkin- duit of communication in Egypt’s internal transport sys- son this volume). -
![روشک [Fa] Egypt Fishery Products [FA] SECTION](https://docslib.b-cdn.net/cover/5909/fa-egypt-fishery-products-fa-section-1885909.webp)
روشک [Fa] Egypt Fishery Products [FA] SECTION
[fa] Validity date from fa] Egypt] روشک 10/08/2007 00063 [FA] SECTION Fishery products [fa] Date of publication 24/11/2019 [fa] List in force fa] Regions [fa] Activities [fa] Remark [fa] Date of request] رهش مان دییات هرامش 02.F.I Jiang's Fish Processor Co., Ltd El Obour City Al Qalyubiyah [fa] PP 17/12/2013 02.F.II ALALAMIA OVER SEAS Badr city Cairo Governorate [fa] PP 01/09/2019 03.F.I NELSON INTERNATIONAL MARINE PRODUCTS (L.L.C) Alexandria Al Iskandariyah [fa] PP 03.F.III Alexandria Co. For Import And Export Fish And Fishery Product Alexandria Al Iskandariyah [fa] PP 09/10/2007 03.F.IV Egyptian European Company For Food Products Alexandria Borg El Arab [fa] PP 09/12/2013 03.F.S.S.S.V Summer Moon New Borg AL Arab Alexandria [fa] PP 16/01/2018 (045.F.I) MACRAY INDUSTRIES New Nobaria City ELBahira Governorate [fa] PP 29/08/2019 045.F.II Happyco Trading And Manufacturing Co Nubaria ELBahira Governorate [fa] PP 11/01/2021 047.F.1 IZAFCO GLOBAL FOR INVESTMENT AND DEVELOPMENT Baltim Kafr ash Shaykh [fa] PP 25/03/2019 047.F.II Nile For Processing, Packing of Foodstuff and Fish Metobus Kafr ash Shaykh [fa] PP 17/08/2020 057.F.I Alandlouse Co. For Fish Processor New Damiatta City Domiatta [fa] PP 09/10/2007 064.F.I M T C for Import and Export Ismailia Al Isma`iliyah [fa] PP 064.F.II El Samah co for trading and exporting fish Ismailia Al Isma`iliyah [fa] PP 064.F.III ELMOHANDES For Save, Freeze And Packing Fish El Qantara El Sharqiya Ismailia [fa] PP 14/02/2019 066.F.I Sala Company For Importation And Exportation Port Said Port Said [fa] PP 1 / 2 [fa] List in force fa] Regions [fa] Activities [fa] Remark [fa] Date of request] رهش مان دییات هرامش 066.F.II King Fish For Investment Port Said Port Said [fa] PP 04/12/2008 066.F.III El Radwan Company for Cooling and Freezing Fish Port Said Port Said [fa] PP 28/01/2015 066.F.IV Golden Dove Company For Fish Port Said Port Said [fa] PP 03/09/2014 066.F.IX National Company For Fisheries East Al Tafreah East Port Said Port Said Port Said [fa] PP 04/04/2021 066.F.V. -

Measuring Services .. You Can Count on
Calibration-Valiadion -Temparature Mapping Measuring Services .. You can count on ... As a growing Company We Look forward to Achieve the difficult equation through providing a high quality services with a competitive cost Our team technical knowledge and experience, coupled with our steadfast commitment to quality and the highest standards of customer service, provide our clients with reliable, accurate and thoroughly documented controlled results, and ensuring optimal equipment performance, documented regulatory and quality compliance, in addition to minimizing equipment and facility down time. About Us Egyptian Engineering and Scientific Services Company provide comprehensive Calibration, Clean room Validation , Temperature Mapping and Qualification services to the pharmaceutical, medical device and Food industries. We Also Supply Handheld Instruments , Data loggers , Measuring and Calibration Instruments , Calibrators and Environmental Monitoring Systems ( temperature , Humidity , Differential Pressure and Particle Counting ) EESS an ISO 9001:2008 certified company in the scope of Calibration, Validation and Thermal Validation Services Market Sectors EESS has experience in process and cleanroom technology and fully understands the needs for manufacturing facilities required by highly regulated and demanding manufacturing industries. Pharmaceutical-Medical Devices-Food and Beverage TECHNICAL SERVICES EESS provide reliable accurate and precise services for our customers with the highest level of quality and professionalism. It is our -
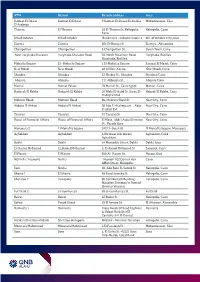
ATM Branch Branch Address Area Gameat El Dowal El
ATM Branch Branch address Area Gameat El Dowal Gameat El Dowal 9 Gameat El-Dewal El-Arabia Mohandessein, Giza El Arabeya Thawra El-Thawra 18 El-Thawra St. Heliopolis, Heliopolis, Cairo Cairo 6th of October 6th of October Banks area - industrial zone 4 6th of October City, Giza Zizenia Zizenia 601 El-Horaya St Zizenya , Alexandria Champollion Champollion 5 Champollion St., Down Town, Cairo New Hurghada Sheraton Hurghada Sheraton Road 36 North Mountain Road, Hurghada, Red Sea Hurghada, Red Sea Mahatta Square El - Mahatta Square 1 El-Mahatta Square Sarayat El Maadi, Cairo New Maadi New Maadi 48 Al Nasr Avenu New Maadi, Cairo Shoubra Shoubra 53 Shobra St., Shoubra Shoubra, Cairo Abassia Abassia 111 Abbassia St., Abassia Cairo Manial Manial Palace 78 Manial St., Cairo Egypt Manial , Cairo Hadayek El Kobba Hadayek El Kobba 16 Waly El-Aahd St, Saray El- Hdayek El Kobba, Cairo Hadayek Mall Makram Ebeid Makram Ebeid 86, Makram Ebeid St Nasr City, Cairo Abbass El Akkad Abbass El Akkad 20 Abo El Ataheya str. , Abas Nasr City, Cairo El akad Ext Tayaran Tayaran 32 Tayaran St. Nasr City, Cairo House of Financial Affairs House of Financial Affairs El Masa, Abdel Azziz Shenawy Nasr City, Cairo St., Parade Area Mansoura 2 El Mohafza Square 242 El- Guish St. El Mohafza Square, Mansoura Aghakhan Aghakhan 12th tower nile towers Aghakhan, Cairo Aghakhan Dokki Dokki 64 Mossadak Street, Dokki Dokki, Giza El- Kamel Mohamed El_Kamel Mohamed 2, El-Kamel Mohamed St. Zamalek, Cairo El Haram El Haram 360 Al- Haram St. Haram, Giza NOZHA ( Triumph) Nozha Triumph.102 Osman Ebn Cairo Affan Street, Heliopolis Safir Nozha 60, Abo Bakr El-Seddik St. -

Hunting and Illegal Killing of Birds Along The
1 Acknowledgements This study was made possible through the collaborative efforts of a number of individuals and organisations. These include: generous financial support from the German Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety provided through the Secretariat of the African-Eurasian Migratory Waterbird Agreement (UNEP/AEWA Secretariat), supervision by the BirdLife international Secretariat; and support implementation by Nature Conservation Egypt and the Nature Conservation Sector - Egyptian Environmental Affairs Agency (providing logistical support to conduct the survey on the ground). This study benefited from the experience of Dr. Sherif Baha El Din, the CEO of NCE and Mr. Waheed Salama (Responsible Hunting Programme Manager, NCE), who provided invaluable mentoring and guidance throughout the project. The study would not have been possible without the contribution of the 73 individuals who agreed to be interviewed during the field survey which formed the main component of the study. I would like to acknowledge the contributions from Park rangers, who provided assistance in the field and helped us to conduct the interviews: Nagwa El Shinawy, Hussein Rashad, Yasser Galal, Moheb Isehak, Zaky M.Zaky and Waleed Atef. I also wish to thank the NCE volunteers, Hashem Morsi and Watter El Bahry, who provided significant assistance during the field survey, including taking the photos that have been used for this report. Special thanks go to Mr Mark Balman from the BirdLife Secretariat’s Information Management team, for contributing his time to draw the map used in this report. I am also grateful to BirdLife International including Claire Thompson, David Thomas and Carol Stork, who reviewed the report in its early stages and provided valuable comments.