Prediction of High-Risk Probability Areas Under Current and Future Climate Scenarios in India for the Establishment of Fall Armyworm Dr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
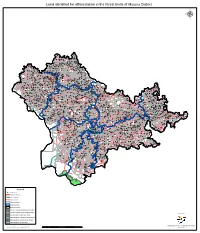
Land Identified for Afforestation in the Forest Limits of Mysuru District Μ
Land identified for afforestation in the forest limits of Mysuru District µ Bettahalli Guluvina Attiguppe Hosuru Hosuru Mudiguppe Munjanahalli Mundur Kallikoppalu Dodda Koppalu Kanchanahalli Chikka Bherya Kallikoppalu Tandre Salekoppalu Tandrekoppalu Sankanahalli Somanahalli Bherya Melur Haradanahali Bachahalli Badiganahalli Madapura Katnalu Kurubarahalli Sugganahalli Abburu Doddadahalli Ramapura Mudlabidu Elemuddanahalli Harumballikoppalu Basavanahalli Kallimuddanahalli Karatalu Lakkikuppe Yaladahalli Kodiyala Manugonahalli Hebsuru Harumballi Lakkikuppekoppalu Ankanahalli Kanagalu Saligrama Hosa Agrahara Sarguru Hale Mirle Tandere Gayanahalli Honnenahalli Kottayanakoppalu Cummanahalli Natnahalli Hanumanahalli Dodda Vaddaragudi Honnapura Alchanahalli Dodda Hanasoge Kanchinakere Pasupati Kakanahalli Subbayyanakoppalu Kedaga Chiknayakanahalli Adaguru PasupatikoppaluMavanuru Mirle Chikka Vaddaragudi Rajanabilugull Bichanahalli Galigekere Balluru C Koppalu Malnayakanahalli Ganganahalli Arjunahalli KD Kambipura Yaremanuganahalli Koluru Ganganahallikoppalu Chikka Hanasage Kaggala Adaguru Halaganahalli Chennamgere Ganganakuppe Kavalanahalli Bandahalli Kalyanapura Sannegaudanakoppalu Dammanahalli Byalakuppe Suragahalli Hadya Baluru Doddakoppalu Harinahalli Sakkare Dod Kamarhalli Kumarikoppalu Karadipura Nadappanahalli K.R.Nagar Badakanakoppalu Anivalu Meluru Halgegaudankoppalu Baluru Chapparadahalli Sangarashettihalli Maraduru Kanaganahalli Ambalare Dammanahallikoppalu Diddahalli Chunchanakatte Chik Kamarhalli Manchinahalli Maradur Koppalu Mayigaudanahalli -

Title Butterflies Collected in and Around Lambir Hills National Park
Butterflies collected in and around Lambir Hills National Park, Title Sarawak, Malaysia in Borneo ITIOKA, Takao; YAMAMOTO, Takuji; TZUCHIYA, Taizo; OKUBO, Tadahiro; YAGO, Masaya; SEKI, Yasuo; Author(s) OHSHIMA, Yasuhiro; KATSUYAMA, Raiichiro; CHIBA, Hideyuki; YATA, Osamu Contributions from the Biological Laboratory, Kyoto Citation University (2009), 30(1): 25-68 Issue Date 2009-03-27 URL http://hdl.handle.net/2433/156421 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University Contn bioL Lab, Kyoto Univ., Vot. 30, pp. 25-68 March 2009 Butterflies collected in and around Lambir Hills National ParK SarawaK Malaysia in Borneo Takao ITioKA, Takuji YAMAMo'rD, Taizo TzucHiyA, Tadahiro OKuBo, Masaya YAGo, Yasuo SEKi, Yasuhiro OHsHIMA, Raiichiro KATsuyAMA, Hideyuki CHiBA and Osamu YATA ABSTRACT Data ofbutterflies collected in Lambir Hills National Patk, Sarawak, Malaysia in Borneo, and in ks surrounding areas since 1996 are presented. In addition, the data ofobservation for several species wimessed but not caught are also presented. In tota1, 347 butterfly species are listed with biological information (habitat etc.) when available. KEY WORDS Lepidoptera! inventory1 tropical rainforesti species diversity1 species richness! insect fauna Introduction The primary lowland forests in the Southeast Asian (SEA) tropics are characterized by the extremely species-rich biodiversity (Whitmore 1998). Arthropod assemblages comprise the main part of the biodiversity in tropical rainforests (Erwin 1982, Wilson 1992). Many inventory studies have been done focusing on various arthropod taxa to reveal the species-richness of arthropod assemblages in SEA tropical rainforests (e.g. Holloway & lntachat 2003). The butterfly is one of the most studied taxonomic groups in arthropods in the SEA region; the accumulated information on the taxonomy and geographic distribution were organized by Tsukada & Nishiyama (1980), Yata & Morishita (1981), Aoki et al. -

Abstracts of 107Th ISC Environmental Sciences
Section VII : Environmental Sciences PROCEEDINGS OF THE 107TH INDIAN SCIENCE CONGRESS Bangalore, 2020 PART II SECTION OF ENVIORNMENTAL SCIENCES President: Dr. Ranbeer Singh Rawal CONTENTS I. Presidential Address 5 II. Abstract of Platinum Jubilee Lecture 27 III. Abstracts of Symposium/Invited Lectures 31 IV. Abstract of Oral Presentations 67 V. Abstract of Poster Presentations 119 VI. List of Past Sectional Presidents 269 VII. Authors Index 273 (1) (2) Section VII : Environmental Sciences 107TH INDIAN SCIENCE CONGRESS January 3-7, 2020, Bangalore I PRESIDENTIAL ADDRESS President: Dr. Ranbeer Singh Rawal (3) (4) Section VII : Environmental Sciences PRESIDENTIAL ADDRESS A01: INTEGRATING SCIENCE & TECHNOLOGY IN RURAL TRANSFORMATION -CASE IN POINT INDIAN HIMALAYAN REGION Ranbeer S. Rawal Director, G.B. Pant National Institute of Himalayan Environment & Sustainable Development (GBPNIHESD), Kosi-Katarmal, Almora 263 643, Uttarakhand Email: [email protected] Dignitaries, colleagues, dear students, ladies and gentlemen, on behalf of the Environmental Sciences Section, thank you for taking the time and effort to join us here in this 107th session of Indian Science Congress. Am privileged and honoured to deliver this presidential address to make a justice with the purpose that has attracted all of us here. My attempt here is not to take a deep-dive in science and technology that is the domain of you all, rather I wish to emphasize upon possibilities of integrating S&T for Rural Transformation. I admit, this address does not include any new idea, but it attempts to highlight that path for improved quality of life of India’s rural people passes through S&T interventions. -

Chapter 4.1.9 Ground Water Resources Thoothukudi District
CHAPTER 4.1.9 GROUND WATER RESOURCES THOOTHUKUDI DISTRICT 1 INDEX CHAPTER PAGE NO. INTRODUCTION 3 THOOTHUKUDI DISTRICT – ADMINISTRATIVE SETUP 3 1. HYDROGEOLOGY 3-7 2. GROUND WATER REGIME MONITORING 8-15 3. DYNAMIC GROUND WATER RESOURCES 15-24 4. GROUND WATER QUALITY ISSUES 24-25 5. GROUND WATER ISSUES AND CHALLENGES 25-26 6. GROUND WATER MANAGEMENT AND REGULATION 26-32 7. TOOLS AND METHODS 32-33 8. PERFORMANCE INDICATORS 33-36 9. REFORMS UNDERTAKEN/ BEING UNDERTAKEN / PROPOSED IF ANY 10. ROAD MAPS OF ACTIVITIES/TASKS PROPOSED FOR BETTER GOVERNANCE WITH TIMELINES AND AGENCIES RESPONSIBLE FOR EACH ACTIVITY 2 GROUND WATER REPORT OF THOOTHUKUDI DISTRICT INRODUCTION : In Tamil Nadu, the surface water resources are fully utilized by various stake holders. The demand of water is increasing day by day. So, groundwater resources play a vital role for additional demand by farmers and Industries and domestic usage leads to rapid development of groundwater. About 63% of available groundwater resources are now being used. However, the development is not uniform all over the State, and in certain districts of Tamil Nadu, intensive groundwater development had led to declining water levels, increasing trend of Over Exploited and Critical Firkas, saline water intrusion, etc. ADMINISTRATIVE SET UP The geographical area of Thoothukudi district is 459054 hectares (4590.54sq.km) accounting for 3.5% of the geographical area of Tamilnadu State. This district has well laid out roads and railway lines connecting all major towns within and outside the State. For administrative purpose this district has been divided into 8 Taluks, 12 Blocks and 41 Firkas. -

Of the United Nations Mission in the DRC / MONUC – MONUSCO
Assessing the of the United Nations Mission in the DRC / MONUC – MONUSCO REPORT 3/2019 Publisher: Norwegian Institute of International Affairs Copyright: © Norwegian Institute of International Affairs 2019 ISBN: 978-82-7002-346-2 Any views expressed in this publication are those of the author. Tey should not be interpreted as reflecting the views of the Norwegian Institute of International Affairs. Te text may not be re-published in part or in full without the permission of NUPI and the authors. Visiting address: C.J. Hambros plass 2d Address: P.O. Box 8159 Dep. NO-0033 Oslo, Norway Internet: effectivepeaceops.net | www.nupi.no E-mail: [email protected] Fax: [+ 47] 22 99 40 50 Tel: [+ 47] 22 99 40 00 Assessing the Efectiveness of the UN Missions in the DRC (MONUC-MONUSCO) Lead Author Dr Alexandra Novosseloff, International Peace Institute (IPI), New York and Norwegian Institute of International Affairs (NUPI), Oslo Co-authors Dr Adriana Erthal Abdenur, Igarapé Institute, Rio de Janeiro, Brazil Prof. Tomas Mandrup, Stellenbosch University, South Africa, and Royal Danish Defence College, Copenhagen Aaron Pangburn, Social Science Research Council (SSRC), New York Data Contributors Ryan Rappa and Paul von Chamier, Center on International Cooperation (CIC), New York University, New York EPON Series Editor Dr Cedric de Coning, NUPI External Reference Group Dr Tatiana Carayannis, SSRC, New York Lisa Sharland, Australian Strategic Policy Institute, Canberra Dr Charles Hunt, Royal Melbourne Institute of Technology (RMIT) University, Australia Adam Day, Centre for Policy Research, UN University, New York Cover photo: UN Photo/Sylvain Liechti UN Photo/ Abel Kavanagh Contents Acknowledgements 5 Acronyms 7 Executive Summary 13 Te effectiveness of the UN Missions in the DRC across eight critical dimensions 14 Strategic and Operational Impact of the UN Missions in the DRC 18 Constraints and Challenges of the UN Missions in the DRC 18 Current Dilemmas 19 Introduction 21 Section 1. -
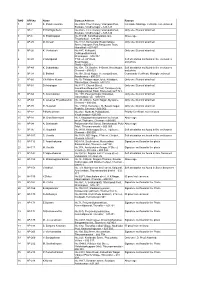
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Democratic Republic of Congo Democratic Republic of Congo Gis Unit, Monuc Africa
Map No.SP. 103 ADMINISTRATIVE MAP OF THE DEMOCRATIC REPUBLIC OF CONGO DEMOCRATIC REPUBLIC OF CONGO GIS UNIT, MONUC AFRICA 12°30'0"E 15°0'0"E 17°30'0"E 20°0'0"E 22°30'0"E 25°0'0"E 27°30'0"E 30°0'0"E Central African Republic N N " " 0 0 ' Sudan ' 0 0 ° ° 5 5 Z o n g oBangui Mobayi Bosobolo Gbadolite Yakoma Ango Yaounde Bondo Nord Ubangi Niangara Faradje Cameroon Libenge Bas Uele Dungu Bambesa Businga G e m e n a Haut Uele Poko Rungu Watsa Sud Ubangi Aru Aketi B u tt a II s ii rr o r e Kungu Budjala v N i N " R " 0 0 ' i ' g 0 n 0 3 a 3 ° b Mahagi ° 2 U L ii s a ll a Bumba Wamba 2 Orientale Mongala Co Djugu ng o R i Makanza v Banalia B u n ii a Lake Albert Bongandanga er Irumu Bomongo MambasaIturi B a s a n k u s u Basoko Yahuma Bafwasende Equateur Isangi Djolu Yangambi K i s a n g a n i Bolomba Befale Tshopa K i s a n g a n i Beni Uganda M b a n d a k a N N " Equateur " 0 0 ' ' 0 0 ° Lubero ° 0 Ingende B o e n d e 0 Gabon Ubundu Lake Edward Opala Bikoro Bokungu Lubutu North Kivu Congo Tshuapa Lukolela Ikela Rutshuru Kiri Punia Walikale Masisi Monkoto G o m a Yumbi II n o n g o Kigali Bolobo Lake Kivu Rwanda Lomela Kalehe S S " KabareB u k a v u " 0 0 ' ' 0 Kailo Walungu 0 3 3 ° Shabunda ° 2 2 Mai Ndombe K ii n d u Mushie Mwenga Kwamouth Maniema Pangi B a n d u n d u Bujumbura Oshwe Katako-Kombe South Kivu Uvira Dekese Kole Sankuru Burundi Kas ai R Bagata iver Kibombo Brazzaville Ilebo Fizi Kinshasa Kasongo KasanguluKinshasa Bandundu Bulungu Kasai Oriental Kabambare K e n g e Mweka Lubefu S Luozi L u s a m b o S " Tshela Madimba Kwilu Kasai -

From Resource War to ‘Violent Peace’ Transition in the Democratic Republic of the Congo (DRC) from Resource War to ‘Violent Peace’
paper 50 From Resource War to ‘Violent Peace’ Transition in the Democratic Republic of the Congo (DRC) From Resource War to ‘Violent Peace’ Transition in the Democratic Republic of Congo (DRC) by Björn Aust and Willem Jaspers Published by ©BICC, Bonn 2006 Bonn International Center for Conversion Director: Peter J. Croll An der Elisabethkirche 25 D-53113 Bonn Germany Phone: +49-228-911960 Fax: +49-228-241215 E-mail: [email protected] Internet: www.bicc.de Cover Photo: Willem Jaspers From Resource War to ‘Violent Peace’ Table of contents Summary 4 List of Acronyms 6 Introduction 8 War and war economy in the DRC (1998–2002) 10 Post-war economy and transition in the DRC 12 Aim and structure of the paper 14 1. The Congolese peace process 16 1.1 Power shifts and developments leading to the peace agreement 17 Prologue: Africa’s ‘First World War’ and its war economy 18 Power shifts and the spoils of (formal) peace 24 1.2 Political transition: Structural challenges and spoiler problems 29 Humanitarian Situation and International Assistance 30 ‘Spoiler problems’ and political stalemate in the TNG 34 Systemic Corruption and its Impact on Transition 40 1.3 ‘Violent peace’ and security-related liabilities to transition 56 MONUC and its contribution to peace in the DRC 57 Security-related developments in different parts of the DRC since 2002 60 1.4 Fragility of security sector reform 70 Power struggles between institutions and parallel command structures 76 2. A Tale of two cities: Goma and Bukavu as case studies of the transition in North and South Kivu -

(ESDD) for 252 MW Wind Power Project in Thoothukudi District, Tamil Nadu
` FINAL REPORT Environmental and Social Due Diligence (ESDD) for 252 MW Wind Power Project in Thoothukudi district, Tamil Nadu By Engie and Vivid Solaire Energy Private Limited Prepared for: L &T Financial Services Prepared by: Arcadis India Private Limited Inc. Langdon & Seah | Hyder Consulting | EC Harris | SENES CONTACTS DR BIPAL JANA Principal Consultant [email protected] Arcadis India Private Limited 3rd Floor, Tower B Logix Techno Park, Sector 127, Noida-201304, India QUALITY ASSURANCE Issue Number Date Prepared By Technical Review Authorised by /Status Mousumi Mondal 001-Draft V.01 Jan 2020 Dr. Bipal Jana Dr. Bipal Jana Bonhisikha Banerjee Mousumi Mondal 002-Draft V.02 Feb 2020 Dr. Bipal Jana Dr. Bipal Jana Bonhisikha Banerjee 003-Draft V.03 Feb 2020 Mousumi Mondal Dr. Bipal Jana Dr. Bipal Jana Mousumi Mondal 004-Final V.01 Mar 2020 Dr. Bipal Jana Dr. Bipal Jana Bonhisikha Banerjee GRM Disclosure Published ESDD report for 252 MW Wind Power Project in Thoothukudi district, Tamil Nadu project of M/s Engie Group conducted by Arcadis India Pvt Ltd engaged by L & T Financial Services. The study was conducted during December 2019. ESDD report was framed according to the E & S guidelines of AIIB. ESDD study has been conducted based on the site visit in and around project region, review of the project related documents/ information, other study reports and stakeholder consultations with Engie representatives, EPC contractor & other contractors, landowners, and others. The interpretation of data and judgment is based on the professional experience and represent professional opinion of the interpreter. The project’s ESDD have been disclosed for public views, comments, and grievances (if any) for the project and project-affected people on the website of L&T Financial Services in English. -

District Survey Report- Thoothukudi District
1 District Survey Report- Thoothukudi District Page Chapter Content No. 1. Introduction 3 2. Overview of Mining Activity in the District 5 3. General Profile of the District 6 4. Geology of Thoothukudi District 10 5. Drainage of Irrigation pattern 16 Land Utilisation Pattern in the District: Forest, Agricultural, 6. 17 Horticultural, Mining etc., 7. Surface Water and Ground Water scenario of the District 18 8. Climate and Rainfall of the District 20 9. Details of Mining Leases in the District 22 10. Details of Royalty or Revenue received in last three years 34 11. Details of Production of Minor Mineral in last three years 35 12. Mineral Map of the District 36 List of Letter of Intent (LOI) Holder in the District along with 13. 37 its validity 14. Total Mineral Reserve available in the district 39 15. Quality/Grade of Mineral available in the district 43 16. Use of Mineral 44 17. Demand and supply of the Mineral in the last three years 44 18. Mining Leases marked on the map of the district 45 Details of the area of where there is a cluster of the mining 19. 47 leases 20. Details of Eco-sensitive area 47 21. Impact on the environment due to Mining activity 49 Remedial measures to mitigate the impact of mining on the 22. 50 environment 23. Reclamation of the mined out area 52 24. Risk assessment & Disaster Management Plan 52 25. Details of Occupational health issue in the District 53 Plantation and Green belt development in respect of leases 26. 54 already granted in the district 27. -

The Political Ecology of a Small-Scale Fishery, Mweru-Luapula, Zambia
Managing inequality: the political ecology of a small-scale fishery, Mweru-Luapula, Zambia Bram Verelst1 University of Ghent, Belgium 1. Introduction Many scholars assume that most small-scale inland fishery communities represent the poorest sections of rural societies (Béné 2003). This claim is often argued through what Béné calls the "old paradigm" on poverty in inland fisheries: poverty is associated with natural factors including the ecological effects of high catch rates and exploitation levels. The view of inland fishing communities as the "poorest of the poorest" does not imply directly that fishing automatically lead to poverty, but it is linked to the nature of many inland fishing areas as a common-pool resources (CPRs) (Gordon 2005). According to this paradigm, a common and open-access property resource is incapable of sustaining increasing exploitation levels caused by horizontal effects (e.g. population pressure) and vertical intensification (e.g. technological improvement) (Brox 1990 in Jul-Larsen et al. 2003; Kapasa, Malasha and Wilson 2005). The gradual exhaustion of fisheries due to "Malthusian" overfishing was identified by H. Scott Gordon (1954) and called the "tragedy of the commons" by Hardin (1968). This influential model explains that whenever individuals use a resource in common – without any form of regulation or restriction – this will inevitably lead to its environmental degradation. This link is exemplified by the prisoner's dilemma game where individual actors, by rationally following their self-interest, will eventually deplete a shared resource, which is ultimately against the interest of each actor involved (Haller and Merten 2008; Ostrom 1990). Summarized, the model argues that the open-access nature of a fisheries resource will unavoidably lead to its overexploitation (Kraan 2011). -

Reservation for Women in Karnataka Gram Panchayats: the Implications Of
Reservation for Women in Karnataka Gram Panchayats: The Implications of Non-Random Reservation and the Effect of Women Leaders Janhavi Nilekani Economics Senior Essay Yale College 2010 Acknowledgements: I am grateful to Professors T.N. Srinivasan and Thad Dunning of Yale University for guidance and support throughout this project. A 2008 Democracy and Development Fellowship from the Whitney and Betty MacMillan Center at Yale University enabled me to collect some of the data on government performance. Reservation data collected by Dunning and Nilekani (2009) was extremely helpful. I thank U. A. Vasanth Rao and the Chief Decentralization Analysis Cell of the Gram Swaraj Project for providing fiscal data. Janhavi Nilekani Table of Contents: Abstract 1. Introduction 1.1. Karnataka’s Panchayati Raj 2. Theoretical Background 2.1. Theoretical Support and Criticisms of Political Reservation for Women 2.2. The Impact of Reservation for Women and Minorities in Indian Panchayats 3. Methodological Critique: Non-random Reservation in Indian States. 3.1. Introduction 3.2. Evidence Against Reservation Being Random in All Indian States 3.3. Data Collection 3.4. The Karnataka Panchayati Raj Reservation Process 3.5. Implications of Karnataka’s Reservation Process 3.6. Conclusion: Misestimation of the Effect of Reservation in Previous Research 4. Random Assignment at the Margin of Reservation 4.1. Data Collection 4.2. Empirical Design 4.3. Results and Discussion 5. Conclusion Works Cited Appendix A: Further Details on the Research Design Appendix B: Rural Development and Panchayati Raj Data Independent T-test Results Appendix C: Gram Swaraj Project Fiscal Data Independent T-test Results 2 Janhavi Nilekani Abstract: Do women leaders of Indian village councils govern differently than their male counterparts? The literature finds that the election of women presidents through electoral quotas shifts public goods provision towards female preferences and improves overall governance.