John Dewolfe Mckinley Watt II
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Population Polygons of Tektite Specific Gravity for Various Localities in Australasia
Reprinted from GEOCHIMICA ET COSMOCHIMICA ACTA Vol. 28, NO. 6,pp. 821-839 t II d 0 i (CA'TECORYI I {NASA CR OR TMX OR AD NUMBER1 ! POPULATION POLYGONS OF TEKTITE SPECIFIC GRAVITY FOR VARIOUS LOCALITIES IN AUSTRALASIA DEANR. CHAPMAN,HOWARD K. LARSONand LEROYC. SCHEIBER N.A.S.A., Ames Research Center, Moffett Field, California PERGAMON PRESS NEWYORK * OXFORD - LONDON - PARIS 1964 Geochimica et Cosmochimica Acta 1984, Val. 28, pp. 821 to 839. Pergamon Press Ltd. Printed in Northern Ireland Population polygons of tektite specific gravity for various localities in Australasia* DEANR. CHAPMAN,HOWARD K. LARSON and LEROYC. SCHEIBER N.A.S.A., Ames Research Ccnter, Moffett Field, California 31- Abstract-~Ieasurements of specific gravity by the method of liquid flotation have been made on about 6000 tektites from 18 different localities in Australasia, from 1 locality in Texas and 2 in Czechoslovakia. Comparison of specific-gravity population polygons for various localities has led to the unanticipated conclusion that the amtralite population in southwest Australia is essentially the same as the philippinite population, rather than the population elsewhere in Australia. The javanite population appears closely related to certain populations in Australia. In several localities the presence of two superimposed populations is demonstrated. The specific- gravity evidence indicates that the Australasian tektites represent a single event comprising many distinguishable clusters, some of which have partially overlapped. INTRODUCTION #e AT the turn of this century, the Austrian geologist SUESS(1900) gave the collective name of “tektites” to three widely separated groups of natural glass objects then known to be scattered over parts of Australia, Czechoslovakia and the island of Billiton. -

X. Paleontology, Biostratigraphy
BIBLIOGRAPHY OF THE GEOLOGY OF INDONESIA AND SURROUNDING AREAS Edition 7.0, July 2018 J.T. VAN GORSEL X. PALEONTOLOGY, BIOSTRATIGRAPHY www.vangorselslist.com X. PALEONTOLOGY, BIOSTRATIGRAPHY X. PALEONTOLOGY, BIOSTRATIGRAPHY ................................................................................................... 1 X.1. Quaternary-Recent faunas-microfloras and distribution ....................................................................... 60 X.2. Tertiary ............................................................................................................................................. 120 X.3. Jurassic- Cretaceous ........................................................................................................................ 161 X.4. Triassic ............................................................................................................................................ 171 X.5. Paleozoic ......................................................................................................................................... 179 X.6. Quaternary Hominids, Mammals and associated stratigraphy ........................................................... 191 This chapter X of the Bibliography 7.0 contains 288 pages with >2150 papers. These are mainly papers of a more general or regional nature. Numerous additional paleontological papers that deal with faunas/ floras from specific localities are listed under those areas in this Bibliography. It is organized in six sub-chapters: - X.1 on modern and sub-recent -

Takysie Lake – the Classic “Pseudometeorite” – Found by Harvey H
Meteorite Times Magazine Contents Paul Harris Featured Articles Accretion Desk by Martin Horejsi Jim’s Fragments by Jim Tobin Bob’s Findings by Robert Verish Micro Visions by John Kashuba Norm’s Tektite Teasers by Norm Lehrman IMCA Insights by The IMCA Team Meteorite of the Month by Editor Tektite of the Month by Editor Terms Of Use Materials contained in and linked to from this website do not necessarily reflect the views or opinions of The Meteorite Exchange, Inc., nor those of any person connected therewith. In no event shall The Meteorite Exchange, Inc. be responsible for, nor liable for, exposure to any such material in any form by any person or persons, whether written, graphic, audio or otherwise, presented on this or by any other website, web page or other cyber location linked to from this website. The Meteorite Exchange, Inc. does not endorse, edit nor hold any copyright interest in any material found on any website, web page or other cyber location linked to from this website. The Meteorite Exchange, Inc. shall not be held liable for any misinformation by any author, dealer and or seller. In no event will The Meteorite Exchange, Inc. be liable for any damages, including any loss of profits, lost savings, or any other commercial damage, including but not limited to special, consequential, or other damages arising out of this service. © Copyright 2002–2016 The Meteorite Exchange, Inc. All rights reserved. No reproduction of copyrighted material is allowed by any means without prior written permission of the copyright owner. Meteorite Times Magazine Oriented Canyon Diablo Meteorite? I hope so. -

Meteorite Impacts, Earth, and the Solar System
Traces of Catastrophe A Handbook of Shock-Metamorphic Effects in Terrestrial Meteorite Impact Structures Bevan M. French Research Collaborator Department of Mineral Sciences, MRC-119 Smithsonian Institution Washington DC 20560 LPI Contribution No. 954 i Copyright © 1998 by LUNAR AND PLANETARY INSTITUTE The Institute is operated by the Universities Space Research Association under Contract No. NASW-4574 with the National Aeronautics and Space Administration. Material in this volume may be copied without restraint for library, abstract service, education, or personal research purposes; however, republication of any portion thereof requires the written permission of the Insti- tute as well as the appropriate acknowledgment of this publication. Figures 3.1, 3.2, and 3.5 used by permission of the publisher, Oxford University Press, Inc. Figures 3.13, 4.16, 4.28, 4.32, and 4.33 used by permission of the publisher, Springer-Verlag. Figure 4.25 used by permission of the publisher, Yale University. Figure 5.1 used by permission of the publisher, Geological Society of America. See individual captions for reference citations. This volume may be cited as French B. M. (1998) Traces of Catastrophe:A Handbook of Shock-Metamorphic Effects in Terrestrial Meteorite Impact Structures. LPI Contribution No. 954, Lunar and Planetary Institute, Houston. 120 pp. This volume is distributed by ORDER DEPARTMENT Lunar and Planetary Institute 3600 Bay Area Boulevard Houston TX 77058-1113, USA Phone:281-486-2172 Fax:281-486-2186 E-mail:[email protected] Mail order requestors will be invoiced for the cost of shipping and handling. Cover Art.“One Minute After the End of the Cretaceous.” This artist’s view shows the ancestral Gulf of Mexico near the present Yucatán peninsula as it was 65 m.y. -

PDF Linkchapter
Index [Italic page numbers indicate major references] A arsenic, 116, 143, 168 brecciation, shock, 225, 231 Ashanti crater, Ghana. See Bosumtwi Brent crater, Ontario, 321 Abitibi Subprovince, 305 crater, Ghana bromine, 137 Acraman depression, South Australia, A thy ris Broodkop Shear Zone, 180 211, 212, 218 gurdoni transversalis, 114 Budevska crater, Venus, 24 geochemistry, 216 hunanensis, 114 Bunyeroo Formation, 209, 210, 219, geochronology, 219 aubrite, 145 220, 221, 222 melt rock, 216, 218 augite, 159 Bushveld layered intrusion, 337 paleomagnetism, 219 Australasian strewn field, 114, 133, See also Acraman impact structure 134, 137, 138, 139, 140, 143, Acraman impact structure, South C 144, 146 Australia, 209 australite, 136, 141, 145, 146 Cabin-Medicine Lodge thrust system, Adelaid Geosyncline, 210, 220, 222 Austria, moldavites, 142 227 adularía, 167 Cabin thrust plate, 173, 225, 226, Aeneas on Dione crater, Earth, 24 227, 231, 232 aerodynamically shaped tektites, 135 B calcite, 112, 166 Al Umchaimin depression, western Barbados, tektites, 134, 139, 142, 144 calcium, 115, 116, 128 Iraq, 259 barium, 116, 169, 216 calcium oxide, 186, 203, 216 albite, 209 Barrymore crater, Venus, 44 Callisto, rings, 30 Algoman granites, 293 Basal Member, Onaping Formation, Cambodia, circular structure, 140, 141 alkali, 156, 159, 167 266, 267, 268, 271, 272, 273, Cambrian, Beaverhead impact alkali feldspar, 121, 123, 127, 211 289, 290, 295, 296, 299, 304, structure, Montana, 163, 225, 232 almandine-spessartite, 200 307, 308, 310, 311, 314 Canadian Arctic, -

Abstracts A-L.Fm
Meteoritics & Planetary Science 41, Nr 8, Supplement, A13–A199 (2006) http://meteoritics.org Abstracts 5367 5372 CHARACTERIZATION OF ASTEROIDAL BASALTS THROUGH ONSET OF AQUEOUS ALTERATION IN PRIMITIVE CR REFLECTANCE SPECTROSCOPY AND IMPLICATIONS FOR THE CHONDRITES DAWN MISSION N. M. Abreu and A. J. Brearley. Department of Earth and Planetary Sciences, P. A. Abell 1, D. W. Mittlefehldt1, and M. J. Gaffey2. 1Astromaterials Research University of New Mexico, Albuquerque, New Mexico 87131, USA. E-mail: and Exploration Science, NASA Johnson Space Center, Houston, Texas [email protected] 77058, USA. 2Department of Space Studies, University of North Dakota, Grand Forks, North Dakota 58202, USA Introduction: Although some CR chondrites show evidence of significant aqueous alteration [1], our studies [2] have identified CR Introduction: There are currently five known groups of basaltic chondrites that exhibit only minimal degrees of aqueous alteration. These achondrites that represent material from distinct differentiated parent bodies. meteorites have the potential to provide insights into the earliest stages of These are the howardite-eucrite-diogenite (HED) clan, mesosiderite silicates, aqueous alteration and the characteristics of organic material that has not angrites, Ibitira, and Northwest Africa (NWA) 011 [1]. Spectroscopically, all been affected by aqueous alteration, i.e., contains a relatively pristine record these basaltic achondrite groups have absorption bands located near 1 and 2 of carbonaceous material present in nebular dust. The CR chondrites are of microns due to the presence of pyroxene. Some of these meteorite types have special significance in this regard, because they contain the most primitive spectra that are quite similar, but nevertheless have characteristics (e.g., carbonaceous material currently known [3]. -

Early Quaternary Global Terrestrial Impact of a Whole Comet in the Australasian Tektite Field, Newest Apparent Evidences Discovery from Thailand and East Asia
CEOSEA '98 Proceedillfl.l, Ceo/. Soc. 11/lZfIZY, I/'a BIlII . .jj, December 1999; p. 555-575 Ninth Regional Congress on Geology, Mineral and GEOSEA '98 Energy Resources of Southeast Asia - GEOSEA '98 17 - 19 August 1998 • Shangri-La Hotel, Kuala Lumpur, Malaysia Early Quaternary global terrestrial impact of a whole comet in the Australasian tektite field, newest apparent evidences discovery from Thailand and East Asia 2 3 SANGAD BUNOPAS!, JOHN T. WASSON , PAUL VELLA , HENRY FONTAINE!, SHIGEKl 5 6 1 HADA\ CLIVE BURRETT , THNA SUPHAJANYA AND SOMBOON KHOSITANONT 1C/O Geo!. Surv. Div., Dept. of Min. Res. Rama IV Rd., Bangkok 10400, Thailand 2lnst. of Geophysics and planetary Physics, University of California Los Angeles, Cal 90024-1567, USA 31 Vella Street, Titahi Bay 6006, Wellington, New Zealand c/o Victoria University, Wellington, N.Z. 4 Geology Department, Research Inst. for Higher Education, Kobe University Nada-ku, Kobe 657, Japan 5School of Earth Sciences & Centre for Mineral Deposit Research, University of Tasmania Hobart, Tasmania 7001 , Australia 6Department of Geology, Chulalongkorn University Bangkok, Thailand Abstract: Further to our current research in layered and splashed tektites, and numerous impact multiple craters in Ubon; our 1997 new discovery evidences in Khorat, both in northeast Thailand, includes the wide continental burns, mass extinction oftrees, ancient elephants, other mammals; and the thick catastrophic loess. All of these were linked to an extraordinary global disaster in a catastrophic event in Thailand and East Asia. These evidences were belonged to and superimposed on the known Australasian tektites strewn field that covers 1/10 of the Earth's surface. -

Pergamon Press
. b' . Reprinted from GEOCHIMICA ET COSMOCHIMICA ACTA Journal of the Geochemical Society PERGAMON PRESS OXFORD LONDON NEW YORK 0 PARIS Qeochimica et Cosmochimica Acta 1964, Vol. 28, pp. 841 to 880. Pergamon Press Ltd. Printed in Northern Ireland On the unity and origin of the Australasian tektites C DEANR. CHAPMAN National Aeronautics and Space Administration, smes Research Center Moffett Field, California Abstract-Aerodynamic experiments with tektite glass demonstrate that, subsequent to the termination of ablation, high thermal stresses are formed within a thin outer shell. This shell spontaneously spalls, leaving a core shaped like many of the tektites found in Billiton, Java, Philippines and Australia. Certain tektite fragments from Java and Australia are shown to have the same internal stress pattern as pieces of aerothermal stress shell. The over-all evidence indicates that the Australasian tektites descended into the atmosphere as rigid objects of glass. Core shapes found in the extreme southwest part of Australia surprisingly are more like those found in the Philippines than in other areas of Australia; comparisons of chemical analyses and of specific gravity population polygons reveal the same striking circumstances. It is concluded that all Australasian tektites represent a single event. A study of tektite forms, in relation to results from laboratory experiments, indicates that the temperature of formation of primary shapes varied progressively across the Australasian strewnfield. The sculpture on certain australites provides a record of tumbling during entry into the Earth’s atmosphere. The effect of turning on the determination of entry trajectories is discussed, and new evidence is presented pertaining to the deceleration which existed when ablation terminated on the australites. -

Human Origin Sites and the World Heritage Convention in Asia
39 World Heritage papers39 World Heritage papers HEADWORLD HERITAGES 3 Human origin sites and the Human origin sites and the Heritage World in Asia Convention World Heritage Convention in Asia For more information contact: UNESCO World Heritage Centre papers 7, place Fontenoy 75352 Paris 07 SP France Tel: 33 (0)1 45 68 24 96 Fax: 33 (0)1 45 68 55 70 E-mail: [email protected] http://whc.unesco.org World HeritageWorld Human origin sites and the World Heritage Convention in Asia Nuria Sanz, Editor Coordinator of the World Heritage/HEADS Programme Published in 2014 by the United Nations Educational, Scientific and Cultural Organization, 7, place de Fontenoy, 75352 Paris 07 SP, France and the UNESCO Office in Mexico, Presidente Masaryk 526, Polanco, Miguel Hidalgo, 11550 Ciudad de Mexico, D.F., Mexico. © UNESCO 2014 ISBN 978-92-3-100043-0 This publication is available in Open Access under the Attribution-ShareAlike 3.0 IGO (CC-BY-SA 3.0 IGO) license (http://creativecommons.org/licenses/by-sa/3.0/igo/). By using the content of this publication, the users accept to be bound by the terms of use of the UNESCO Open Access Repository (http://www.unesco.org/open-access/terms-use-ccbysa-en). The designations employed and the presentation of material throughout this publication do not imply the expression of any opinion whatsoever on the part of UNESCO concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The ideas and opinions expressed in this publication are those of the authors; they are not necessarily those of UNESCO and do not commit the Organization. -

Population Polygons of Tektite Specific Gravity for Various Localities in Australasia
https://ntrs.nasa.gov/search.jsp?R=19640021088 2020-03-24T06:18:47+00:00Z Reprinted from GEOCHIMICA ET COSMOCHIMICA ACTA Vol. 28, NO. 6,pp. 821-839 t II d 0 i (CA'TECORYI I {NASA CR OR TMX OR AD NUMBER1 ! POPULATION POLYGONS OF TEKTITE SPECIFIC GRAVITY FOR VARIOUS LOCALITIES IN AUSTRALASIA DEANR. CHAPMAN,HOWARD K. LARSONand LEROYC. SCHEIBER N.A.S.A., Ames Research Center, Moffett Field, California PERGAMON PRESS NEWYORK * OXFORD - LONDON - PARIS 1964 Geochimica et Cosmochimica Acta 1984, Val. 28, pp. 821 to 839. Pergamon Press Ltd. Printed in Northern Ireland Population polygons of tektite specific gravity for various localities in Australasia* DEANR. CHAPMAN,HOWARD K. LARSON and LEROYC. SCHEIBER N.A.S.A., Ames Research Ccnter, Moffett Field, California 31- Abstract-~Ieasurements of specific gravity by the method of liquid flotation have been made on about 6000 tektites from 18 different localities in Australasia, from 1 locality in Texas and 2 in Czechoslovakia. Comparison of specific-gravity population polygons for various localities has led to the unanticipated conclusion that the amtralite population in southwest Australia is essentially the same as the philippinite population, rather than the population elsewhere in Australia. The javanite population appears closely related to certain populations in Australia. In several localities the presence of two superimposed populations is demonstrated. The specific- gravity evidence indicates that the Australasian tektites represent a single event comprising many distinguishable clusters, some of which have partially overlapped. INTRODUCTION #e AT the turn of this century, the Austrian geologist SUESS(1900) gave the collective name of “tektites” to three widely separated groups of natural glass objects then known to be scattered over parts of Australia, Czechoslovakia and the island of Billiton. -
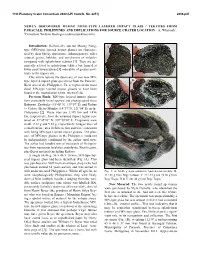
Newly Discovered Muong Nong-Type Layered Impact Glass / Tektites from Paracale, Philippines and Implications for Source Crater Location
11th Planetary Crater Consortium 2020 (LPI Contrib. No. 2251) 2006.pdf NEWLY DISCOVERED MUONG NONG-TYPE LAYERED IMPACT GLASS / TEKTITES FROM PARACALE, PHILIPPINES AND IMPLICATIONS FOR SOURCE CRATER LOCATION. A. Whymark1, 1Consultant Wellsite Geologist ([email protected]). Introduction: Ballistically ejected Muong Nong- A B type (MN-type) layered impact glasses are character- ized by their blocky appearance, inhomogeneity, relict mineral grains, bubbles, and enrichment of volatiles compared with splash-form tektites [1]. They are ge- nerically related to splash-form tektites but formed at lower peak temperatures [1] indicative of greater prox- imity to the impact site. This article reports the discovery of two new MN- type layered impact glass specimens from the Paracale, C D Bicol area of the Philippines. These represent the most distal MN-type layered impact glasses to have been found in the Australasian tektite strewn field. Previous Finds: MN-type layered impact glasses have previously been reported and photographed from Babuyan, Zambales (15°48' N, 119°57' E) and Kubao (= Cubao, Metro Manila) (14°37' N, 121°04' E) in the Philippines [2]. These sites are 1,309 km and 1,456 km, respectively, from the assumed impact region cen- E tered on 17°45'20" N, 107°50'30" E. Fragments were small: 2.12 g and 9.52 g, respectively. Images were of a small surface area in thin section and were consistent with being MN-type layered impact glasses. The pres- ence of MN-type glasses in the Philippines could not be independently confirmed by the author until now. The author had handled tens of thousands of Philippin- ites from numerous localities, mainly the Bicol region, plus Beyer material (including Kubao). -

Geochemical Studies of Tektites from East Asia
Geochemical Journal, Vol. 38, pp. 1 to 17, 2004 Geochemical studies of tektites from East Asia YUNG-TAN LEE,1 JU-CHIN CHEN,1* KUNG-SUAN HO2 and WEN-SHING JUANG2 1Institute of Oceanography, National Taiwan University, Taipei, Taiwan 10617, R.O.C. 2Department of Geology, National Museum of Natural Science, Taichung, Taiwan 40419, R.O.C. (Received March 3, 2003; Accepted June 18, 2003) Thirty tektites from East Asia (including Wenchang and Penglei of Hainan Island, Maoming of Guandong, China; Khon-Kaen of Thailand; Bao Loc of Vietnam; Rizal of Luzon, Philippines) have been analyzed for major and trace ele- ment contents and Rb-Sr isotopic compositions. All the samples studied are splash form tektites. The trace element ratios Ba/Rb (avg. 3.74), Th/Sm (avg. 2.31), Sm/Sc (avg. 0.43), Th/Sc (avg. 0.99) and the rare earth elements (REE) contents of tektites studied are similar to the average upper continental crust. The chemical data of tektites in this study indicate that they were derived from similar target rocks which may be related to post-Archean upper crustal materials. The tektites from East Asia have high positive εSr(0) values-ranging from 164.2 to 198.6, indicating that they were not dominantly derived from recent young sediments, such as soil or loess. The Ar-Ar ages (736.8 ± 55.5~814.6 ± 24.4 ka) of tektites of the present study are consistent with the age of other Australasian tektites, which indicates that all Australasian tektites were derived from a single impact event.