The Macrophage Iron Signature in Health and Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Photon Diagnostics at the FLASH Thz Beamlinethis Article
research papers Photon diagnostics at the FLASH THz beamline1 Rui Pan,a Ekaterina Zapolnova,a Torsten Golz,a Aleksandar J. Krmpot,b ISSN 1600-5775 Mihailo D. Rabasovic,b Jovana Petrovic,c,d Vivek Asgekar,e Bart Faatz,a Franz Tavella,f Andrea Perucchi,g Sergey Kovalev,h Bertram Green,h Gianluca Geloni,i Takanori Tanikawa,i Mikhail Yurkov,a Evgeny Schneidmiller,a Michael Genschj,k and Nikola Stojanovica* Received 17 October 2018 a b Accepted 11 March 2019 Deutsches Elektronen-Synchrotron (DESY), Notkestrasse 85, D-22607 Hamburg, Germany, Institute of Physics Belgrade, Pregrevica 118, 11080 Belgrade, Serbia, cVinca Institute of Nuclear Sciences, Belgrade, Serbia, dCenter for Free-Electron Laser Science, Deutsches Elektronen-Synchrotron (DESY), Notkestrasse 85, D-22607 Hamburg, Germany, eDepartment of Physics, S. P. Pune University, Pune, India, fSLAC National Accelerator Laboratory, Menlo Park, Edited by M. Yabashi, RIKEN SPring-8 Center, California, USA, gElettra – Sincrotrone Trieste SCpA, 34149 Basovizza, Trieste, Italy, hHelmholtz-Zentrum Dresden- Japan Rossendorf (HZDR), Bautzner Landstraße 400, 01328 Dresden, Germany, iEuropean XFEL, Holzkoppel 4, 22869 Schenefeld, Germany, jGerman Aerospace Center (DLR), Institute of Optical Sensor Systems, Rutherfordstraße 2, 1 This article will form part of a virtual special 12489 Berlin, Germany, and kInstitute of Optics and Atomic Physics, Technical University of Berlin, Strasse des issue on X-ray free-electron lasers. 17 Juni 135, 10623 Berlin, Germany. *Correspondence e-mail: [email protected] Keywords: FLASH; intense THz; THz diagnostic; electro-optic; FTIR. The THz beamline at FLASH, DESY, provides both tunable (1–300 THz) narrow-bandwidth (10%) and broad-bandwidth intense (up to 150 uJ) THz pulses delivered in 1 MHz bursts and naturally synchronized with free-electron laser X-ray pulses. -

International Research and Exchanges Board Records
International Research and Exchanges Board Records A Finding Aid to the Collection in the Library of Congress Prepared by Karen Linn Femia, Michael McElderry, and Karen Stuart with the assistance of Jeffery Bryson, Brian McGuire, Jewel McPherson, and Chanté Wilson-Flowers Manuscript Division Library of Congress Washington, D.C. 2011 International Research and Exchanges Board Records Page ii Collection Summary Title: International Research and Exchanges Board Records Span Dates: 1947-1991 (bulk 1956-1983) ID No: MSS80702 Creator: International Research and Exchanges Board Creator: Inter-University Committee on Travel Grants Extent: 331,000 items; 331 cartons; 397.2 linear feet Language: Collection material in English and Russian Repository: Manuscript Division, Library of Congress, Washington, D.C. Abstract: American service organization sponsoring scholarly exchange programs with the Soviet Union and Eastern Europe in the Cold War era. Correspondence, case files, subject files, reports, financial records, printed matter, and other records documenting participants’ personal experiences and research projects as well as the administrative operations, selection process, and collaborative projects of one of America’s principal academic exchange programs. International Research and Exchanges Board Records Page iii Contents Collection Summary .......................................................... ii Administrative Information ......................................................1 Organizational History..........................................................2 -

An X-Ray Gas Monitor for Free-Electron Lasers1
research papers An X-ray gas monitor for free-electron lasers1 Andrey A. Sorokin,a,b* Yilmaz Bican,a Susanne Bonfigt,a Maciej Brachmanski,a ISSN 1600-5775 Markus Braune,a Ulf Fini Jastrow,a Alexander Gottwald,c Hendrik Kaser,c Mathias Richterc and Kai Tiedtkea* aDeutsches Elektronen-Synchrotron (DESY), Notkestrasse 85, D-22607 Hamburg, Germany, bIoffe Physico-Technical Institute, Polytekhnicheskaya 26, 194021 St Petersburg, Russian Federation, and cPhysikalisch-Technische Bundesanstalt Received 21 January 2019 (PTB), Abbestrasse 2–12, D-10587 Berlin, Germany. *Correspondence e-mail: [email protected], Accepted 15 April 2019 [email protected] A novel X-ray gas monitor (XGM) has been developed which allows the Edited by M. Zangrando, IOM-CNR and Elettra-Sincrotrone, Italy measurement of absolute photon pulse energy and photon beam position at all existing and upcoming free-electron lasers (FELs) over a broad spectral range 1 This article will form part of a virtual special covering vacuum ultraviolet (VUV), extreme ultraviolet (EUV) and soft and issue containing papers presented at the hard X-rays. The XGM covers a wide dynamic range from spontaneous PhotonDiag2018 workshop. undulator radiation to FEL radiation and provides a temporal resolution of better than 200 ns. The XGM consists of two X-ray gas-monitor detectors Keywords: free-electron lasers; vacuum (XGMDs) and two huge-aperture open electron multipliers (HAMPs). The ultraviolet; soft X-rays; hard X-rays; photon diagnostics. HAMP enhances the detection efficiency of the XGM for low-intensity radiation down to 105 photons per pulse and for FEL radiation in the hard X-ray spectral range, while the XGMD operates in higher-intensity regimes. -
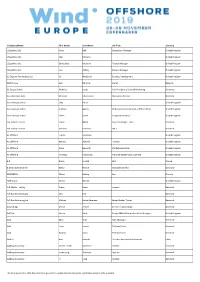
Company Name First Name Last Name Job Title Country
Company Name First Name Last Name Job Title Country 1StopWind Ltd Arran Bell Operations Manager United Kingdom 1StopWind Ltd. Alan Mckerns United Kingdom 1StopWind Ltd. Bernadette McAulay Finance Manager United Kingdom 1StopWind Ltd. Joel Telling General Manager United Kingdom 23 Degrees Renewables Ltd Ed Woodrow Business Development United Kingdom 24SEA bvba Gert De Sitter Owner Belgium 3S Europe GmbH Matthias Lamp Vice President of Sales & Marketing Germany 3sun Denmark ApS Christian Christensen Operations Director Denmark 3sun Group Limited Jody Potter United Kingdom 3sun Group Limited Graham Hacon VP Business Development, Offshore Wind United Kingdom 3sun Group Limited Sherri Smith Company Secretary United Kingdom 3W Industri Service Simon Øland Project manager - sales Denmark 3W Industri Service Kenneth Pedersen IWI-S Denmark 4C Offshore Lauren Anderson United Kingdom 4C Offshore Richard Aukland Director United Kingdom 4C Offshore Rosie Haworth Market Researcher United Kingdom 4C Offshore Vincenzo Poidomani Principal Geotechnical Engineer United Kingdom 8.2 Bruno ALLAIN CEO France 8.2 Monitoring GmbH Bernd Höring Managing director Germany 920338402 Ellinor Meling Ceo Norway A&P Group Emma Harrick United Kingdom A.P. Møller Holding Simon Ibsen Investor Denmark A/S Dan-Bunkering Ltd. Jens Kirk Denmark A/S Dan-Bunkering Ltd. Michael Brunø-Sørensen Senior Bunker Trader Denmark A1wind Aps Martin Jensen Director / A1wind Aps Denmark AAF Ltd Steven Brett Europe MFAS Aftermarket Sales Manager United Kingdom AAG Allan Tarp Sales Manager Denmark -

2018 Annual Report 2018 Valedictorian Address
2018 Annual Report 2018 Valedictorian address Every spring, Life House holds the Celebration of Success fundraising event. This event honors youth who have made significant progress on their personal goals, ranging from completing their GED to maintaining sobriety to keeping employment. One youth is selected to give a short speech to the 250 community members who attend. Below are words from this year’s honoree. I never thought I’d come this far. When I first came to Life House I was homeless, broke and hope- less. The moment I walked through the door, hope hit me like a smack to the face in the form of staff that welcomed me with open arms. Within a month, they helped me get a place of my own and that began my start to a new life. Within a year, I decided to go to college at LSC. I’m proud to say that I have been surprisingly on the Dean’s list twice. I am also proud to say I just finished my fifth semester, but I still have a long way to go. I couldn’t have done it without the support and guidance of Life House. They gave me hope when I thought I had none, they became much more than staff, they became what I call my second family. Without them, I don’t know where I would be at this very moment. Which brings me to my final take away. A poem that I wrote not too long ago titled: “STAY OR GO?” that I thought unintention- ally fit this moment perfectly. -

Dean's List | Illinois Students
Univeristy of Illinois at Urbana-Champaign | Fall 2011 | Dean's List | Illinois Students Middle Student City ZIP Last Name First Name College Major Name Class Addison Adams Sarah Elizabeth 3 Agricultural, Consumer & Environmental Sciences Animal Sciences Addison Cruce Justin T 1 Engineering Civil Engineering Addison Foster Kayla E 1 Liberal Arts & Sciences Biology Addison Gerth Christopher M 2 Engineering Electrical Engineering Addison Kelly Rebecca R 1 Agricultural, Consumer & Environmental Sciences Food Science & Human Nutrition Addison Kwee Dustin J 1 Business Curric Unassigned Addison Patel Megh J 1 Liberal Arts & Sciences Molecular and Cellular Biology Addison Ramir Tyler J 2 Division of General Studies Undeclared Addison Ramirez Amanda Marie 4 Liberal Arts & Sciences Psychology Addison Rowley Jennifer Marie 2 Liberal Arts & Sciences English Addison Saporito Daniela Clara 4 Liberal Arts & Sciences Communication Algonquin Badmus Oyindamola Hanna Y2 Law Law Algonquin Blunk Melissa Kelly 3 Applied Health Sciences Recreation, Sport & Tourism Algonquin Demetriou Nicholas P 1 Engineering Materials Science & Engineering Algonquin Dobbelaere Andie V 2 Business Marketing Algonquin Dombrowski Anthony J 3 Fine & Applied Arts Architectural Studies Algonquin Gardeck Matthew John 4 Business Accountancy Algonquin Kaczar Brian C 1 Business Curric Unassigned Algonquin Kale Brett W 1 Business Curric Unassigned Algonquin Koniewicz Kristen L 2 Liberal Arts & Sciences Biology Algonquin Kunzweiler Christopher K 4 Business Accountancy Algonquin Lindgren -

Active Warrants 07-01-21
LAKE HAVASU POLICE DEPARTMENT 09/01/2021 09:15 Active Warrants By City - LAKE HAVASU CITY Want ID Issued Name (Last, First, Middle) DOB Race Sex Charge Address 14134 06/22/2017 Abeyta, Kristina Jean 10/04/1978 W F Rule Of Criminal Procedure Failure 2511 Mcculloch Blvd N 102, Lake Havasu City To Comply 30006 12/19/2018 Abney, Lance Marcus 08/11/1987 W M Failure To Appear 2nd Deg 3085 El Dorado Ave N, Lake Havasu City 20888 03/24/2018 Abrego, Ruben Arthur Ii 08/15/1976 W M Rule Of Criminal Procedure Failure 450 Mohican Dr, Lake Havasu City To Comply 8244 01/27/2015 Achterberg, Kimberly Dawn 09/11/1982 W F Rule Of Criminal Procedure Failure 100 Lakemaster Ln, Lake Havasu City To Comply 20886 03/24/2018 Ackerman, Steve 08/22/1981 W M Rule Of Criminal Procedure Failure 4062 Gold Spring Rd E, Lake Havasu City To Comply 8764 08/21/2015 Acret, James Elvero 05/08/1963 W M Rule Of Criminal Procedure Failure 2192 Barranca Dr, Lake Havasu City To Comply 33861 05/10/2019 Adams, Joshua Paul 05/12/1986 W M Rule Of Criminal Procedure Failure 660 Grand Island Dr, Lake Havasu City To Comply 8029 11/17/2014 Adams, Kevin Todd 09/27/1979 W M Rules Of Criminal Procedure -failure 2035 Acoma Blvd W .119, Lake Havasu City To Appear 8173 01/06/2015 Adams, Kevin Todd 09/27/1979 W M Rule Of Criminal Procedure Failure 2035 Acoma Blvd W .119, Lake Havasu City To Co 8169 12/30/2014 Adams, Shawn Michael 04/17/1979 W M Rule Of Criminal Procedure Failure 700 Mcculloch Blvd S, Lake Havasu City To Co 15734 10/03/2017 Adcock, Katrina Marie 02/27/1979 W F Failure To Appear -

Mutilation: the Fate of Eastern European Names in America by William F
Mutilation: the Fate of Eastern European Names in America by William F. Hoffman 8 Terrace Dr., Bethel, CT 06801-2102, e-mail: [email protected] Ever since I wrote Polish Surnames: Origins & Meanings, I have received a steady flow of letters and e-mail notes asking about Eastern European names (not just Polish!), either of persons (first names and surnames) or places. I can divide these questions into two basic categories: 1) the name is reasonably correct as given, and the challenge is to find out something about it; and 2) the name has been mangled somewhere along the way, and before it’s possible to learn anything about it, the original form has to be determined. The first category is a piece of cake compared to the second; if I have a reasonably correct form to work with, I either have something on the name or I don’t. A few minutes of searching through my books is usually enough to tell me which is true. But if the name is mangled so badly I can’t even tell what it was originally, there’s no way to proceed. I have to respond by saying, “Sorry, I can’t help you. Do some research, and get back to me when you have a more reliable form of the name.” I’ve learned the hard way that this is the way to go. There have been occasions when someone has submitted a bizarre-looking name, say, Nrawpulkowski, and I spent hours trying to come up with some brilliant explanation of what it might mean, only to hear back, “Gee, sorry, the day after I wrote you I found out it was really Nowakowski. -

Naturalization Index (1904-1958)
Naturalization Index (1904-1958) SURNAME FIRST NAMEMid Name VOL PAGE ADDRESS DATE ERTIFICATE MEMO Abbots William Lovatt 2 13 Buffington Twp 22-Feb-04 Auden Albert 2 47 Glen Campbell 19-Mar-04 Auden Alfred 2 81 Glen Campbell 19-Mar-04 Arur Pul 2 93 Glen Campbell 19-Mar-04 Alik Frank 2 109 Glen Campbell 28-Mar-04 Elik Anderson Andrew 2 149 Ernest 06-Oct-04 Areco Nicholas 2 175 Rossiter 18-Feb-06 Azzara Gaetano 1 7 Clymer 20-Aug-07 13972 Anderson Alfred 2A 16 Dixonville 16-Aug-10 46093 Adamson Charles 2A 33 Wehrum Denied (Later admitted) Adamson Charles 2A 45 Wehrum 27-Aug-11 192257 Adelburg Harry 2A 60 Iselin 13-Feb-12 236264 Alexander, John 2A 71 Dixonville 13-Feb-12 236268 Anderson Alex 3 12 Iselin 11-Feb-13 302444 Augustine Frank 3 98 Glen Campbell 19-Aug-13 406492 Adams John 4 5 Tunnelton 19-Aug-13 359826 Astalos John 4 35 Saltsburg 10-Feb-14 460511 Artzmowicz Frank 4 64 Clymer 18-Aug-14 460544 Andrasain Joseph 4 87 Saxman 09-Feb-15 516121 Anderson Abbonizio Consiglio 5 55 Tunnelton 04-Aug-15 501713 Abruzzi Massimo 5 100 Tunnelton Cancelled Alaimo Angelo 6 26 Chambersville 02-Feb-16 504783 Alimo Adair Hans, Jr. 6 67 Ernest 02-Aug-16 731877 Amantea Antonio 6 72 Heilwood 02-Aug-16 731872 Amerando Leonardo 7 2 McIntyre 15-Feb-17 735426 Abraham John 7 20 Clymer Spoiled petition Allesandro Vito 7 72 Chambersville 01-Aug-17 737682 Antonucci Donato 7 74 Strangford Denied Adamcik Imrich 8 24 Blairsville 05-May-20 1330954 Annunis Matt 8 43 Ernest 07-Feb-18 828258 Arvins Arvins Matt 8 43 Ernest 07-Feb-18 828258 Annunis Acciaccaferro Francesco -

SEACON 79 PROGRESS REPORT No
SEACON 79 PROGRESS REPORT No. 2 SUMMER 1978 37th World Science Fiction Convention METROPOLE HOTEL. BRIGHTON. U.K. Permanent address: SEACON '79 AUGUST 23rd-27 th 1979 14 HENRIETTA ST, LONDON WC2E 8QJ.U.K PRESS MEMBERSHIP EXPLOSION! The first and best piece of SEACON '79 news, and one we might be forgiven for crowing over, is that to date (1st June, 1978) our membership is in excess of 1,8j0. This represents massive international support for our convention, especially from North America. We thank you all. Towards the end of this Progress Report you'll find a full and up-to-date alphabetical listing of our members and their status, NEWS; plus a personal message from our Membership Secretary, Eve Harvey. As Eve points out our membership at the moment exceeds that of both Suncon and Iguanacon at a corresponding stage, and with more than twelve months in hand certainly augurs well for August 1979- ACCOMMODATION BOOKING October 1st is the nominal date upon which booking forms for the SEACON Metropole and other convention hotels will be posted. It's very important of course that our members return these forms promptly so that we can get as many rooms tied up as possible before the end- of-tne-year booking rush for the 1979 holiday season (Brighton is one of Britain's most popular holiday resorts, after all). Bookings for accommodation will be handled by a joint arrangement between Seacon '79 and the official Brighton Accommodation Bureau and no bookings for the convention period will be accepted except through this system. -

029 001 001 DATE FILED Month Day Year MAR 15 1949 PA MAY 14
- From 1942 thru June 1984 — Prince George's Conntg, Md. PAGE General Index to Equity Records MADE BY THE COTT INDEX COMPANY, COLUMBUS, OHIO COTT DATA PROCESSED INDEXES COTTCO FAMILY NAME DIRECTORY DR-DOMESTIC RECORD DEC-DECREE RECORD Eatry Shaat \ AP CODES H-HUSBAND W-WIFE O-OTHER CH-CHANCERY RECORD #—ADOPTION & GUARDIANSHIP DOCKETS ( 029 001 001 R MMlIlltolloa Ha. / DEFENDANTS CASE OBJECT OF BILL DATE FILED — ap! PLAINTIFFS NUMBER CIVEH NAMES GIVEN NAMES Month Day Year SURNAME A - K L - Z ESPEY TRS ROBT G CARTER 4 H CLAY A-2082 FORECLOSE 0 T MAR 15 1949 P A C DRIVE IN CAFE TOWN OF EOMONSTON 12237 TAX SALE MAY 14 1942 P & G ENGINEERING CO FORECLOSE R OF REOEHP LUTHER M CRAMER A-5347 JUN 25 1952 P 1 G ENGINEERING CO INC FORECLOSE R OF REOEHP LUTHER W CRAMER A-5347 JUN 25 1952 P & G ENGINEERING CONSTRUCTION CO INC INJUNCTION 0 HERBERT L REISKIN 0 D-2407 SEP 7 1967 PA H INVESTMENT CO INC T/A ACCOUNTING ETC 0 JOSEPH D£ MICHELE 0 D-1346 MAR 14 1967 P 4 M DRY CLEANERS 4 LAUNDRY RESCISSION OF CONTRACT THE GAME ROOM INC E-84-0903 JUN 6 1984 P 4 S VENDING CO INC ADMINISTRATIVE ORDER PR GEO CO HO E-3261 OCT 21 1977 P £ C CONSTRUCTION CO REMOVE CLOUD ON TITLE WILLIS L JOHNSON E-5947 FEB 26 1979 P £ P INVOLUNTARY DISSOLUTION ETC WILLIAM M SMART £-84-0966 JUN 18 1984 P ( TITLE CQRP FORECLOSE 0 T MARTIN D KR ALL SUB TRS D-85QB MAR 14 1974 P I CORP FORECLOSE D T THELMA A TALMAN TRS 0-6056 APR 21 1971 P CORP PR GEOS CO MD D-9707 FOREC RT/REOEMPT MAY 13 1975 P CORP FOREC RT/REOEMPT DAVID T DAWSON £-6431 MAY 8 1979 P J CORP / ? FOREC RT/REDEMPT THEODORE -
Index to St. Louis, Missouri Naturalization Records Created After Sept
Index to St. Louis, Missouri Naturalization Records Created after Sept. 27, 1906 Alphabetical surname index N–R History & Genealogy Department St. Louis County Library 1640 S. Lindberg Blvd. St. Louis, Missouri 63131 314-994-3300, ext. 2070 [email protected] Index to St. Louis, Missouri Naturalization Records Created after Sept. 27, 1906 This index covers St. Louis, Missouri naturalization records created between October 1, 1906 and December 1928 and is based on the following sources: • Naturalizations, U.S. District Court—Eastern Division, Eastern Judicial District of Missouri, Vols. 1 – 82 • Naturalizations, U.S. Circuit Court— Eastern Division, Eastern Judicial District of Missouri, Vols. 5 – 21 Entries are listed alphabetically by surname, then by given name, and then numerically by volume number. Abbreviations and Notations SLCL = History and Genealogy Department microfilm number (St. Louis County Library) FHL = Family History Library microfilm number * = spelling taken from the signature which differed from name in index. How to obtain copies Photocopies of indexed articles may be requested by sending an email to the History and Genealogy Department at [email protected]. A limit of three searches per request applies. Please review the library's lookup policy at https://www.slcl.org/genealogy-and-local- history/services. A declaration of intention may lead to further records. For more information, contact the National Archives at the address below. Include all information listed on the declaration of intention. National Archives, Central Plains Region 400 W. Pershing Rd. Kansas City, MO 64108 (816) 268-8000 [email protected] History Genealogy Dept. Index to St. Louis, Missouri Naturalization Records St.