Development of Process for Purification of Α and Β-Vetivone from Vetiver Essential Oil & Investigation of Effects of Heavy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Briefing on Carbon Dioxide Specifications for Transport 1St Report of the Thematic Working Group On: CO2 Transport, Storage
Briefing on carbon dioxide specifications for transport 1st Report of the Thematic Working Group on: CO2 transport, storage and networks Release Status: FINAL Author: Dr Peter A Brownsort Date: 29th November 2019 Filename and version: Briefing-CO2-Specs-FINAL-v1.docx EU CCUS PROJECTS NETWORK (No ENER/C2/2017-65/SI2.793333) This project is financed by the European Commission under service contract No. ENER/C2/2017-65/SI2.793333 1 About the CCUS Projects Network The CCUS Projects Network comprises and supports major industrial projects underway across Europe in the field of carbon capture and storage (CCS) and carbon capture and utilisation (CCU). Our Network aims to speed up delivery of these technologies, which the European Commission recognises as crucial to achieving 2050 climate targets. By sharing knowledge and learning from each other, our project members will drive forward the delivery and deployment of CCS and CCU, enabling Europe’s member states to reduce emissions from industry, electricity, transport and heat. http://www.ccusnetwork.eu/ © European Union, 2019 No third-party textual or artistic material is included in the publication without the copyright holder’s prior consent to further dissemination by other third parties. Reproduction is authorised provided the source is acknowledged. This project is financed by the European Commission under service contract No. ENER/C2/2017-65/SI2.793333 2 (Intentionally blank) This project is financed by the European Commission under service contract No. ENER/C2/2017-65/SI2.793333 3 Executive summary There are two types of specification generally relevant to carbon dioxide (CO2) transport, the product specification for end use and the requirement specification for transport. -

Patchouli Essential Oil Extracted from Pogostemon Cablin (Blanco) Benth
Advances in Environmental Biology, 8(7) May 2014, Pages: 2301-2309 AENSI Journals Advances in Environmental Biology ISSN-1995-0756 EISSN-1998-1066 Journal home page: http://www.aensiweb.com/aeb.html Characterization and Antimicrobial Activity of Patchouli Essential Oil Extracted From Pogostemon cablin [Blanco] Benth. [lamiaceae] Ahmad Karimi Ph.D. in pharmacy, University of Santo Tomas, Philippines ARTICLE INFO ABSTRACT Article history: The physico-chemical properties of Philippine patchouli oil, hydro-distilled from fresh Received 25 March 2014 leaves and young shoots of Pogostemon cablin were characterized and found to be Received in revised form 20 April within the specifications set by the United States Essential Oils Society. Philippine 2014 patchouli oil and commercial patchouli oil have the same major components as shown Accepted 15 May 2014 by GC-MS analyses: patchouli alcohol, d-guaiene, a-guaiene, a-patchoulene, Available online 10 June 2014 seychellene, [3-patchoulene, and transcaryophylene, with slightly lower concentrations in the Philippine oil. Using the disk diffusion method patchouli oil was found to be Key words: active against the gram-positive bacteria: Staphylococcus, Bacillus, and Streptococcus Pogostemon cablin, patchouli oil, species. Fifty five percent [11/20] of community and only 14.8% [9/61] of hospital- essential oil, antimierobial activity, Staphylococcus aureus isolates were susceptible to an MIC of 0.03% [v/v.] and Sixty- physico-chemical properties four percent or 23/36 of methicillin-resistant Staphylococcus aureus [MRSA] isolates was sensitive to patchouli oil at 0.06%, as opposed to only 44% or 11/25 of the sensitive strains. Philippine patchouli essential oil was also active against several dermatophytes at 0.25%. -

Carbon Dioxide (Refrigerated Liquid)
Carbon dioxide, refrigerated liquid Safety Data Sheet P-4573 This SDS conforms to U.S. Code of Federal Regulations 29 CFR 1910.1200, Hazard Communication. Date of issue: 01/01/1997 Revision date: 01/30/2021 Supersedes: 09/08/2020 Version: 2.0 SECTION: 1. Product and company identification 1.1. Product identifier Product form : Substance Substance name : Carbon dioxide, refrigerated liquid CAS-No. : 124-38-9 Formula : CO2 Other means of identification : Liquiflow Liquid Carbon Dioxide, Medipure Liquid Carbon Dioxide 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : Medical applications. Industrial use Food applications. 1.3. Details of the supplier of the safety data sheet Linde Inc. 10 Riverview Drive Danbury, CT 06810-6268 - USA 1.4. Emergency telephone number Emergency number : Onsite Emergency: 1-800-645-4633 CHEMTREC, 24hr/day 7days/week — Within USA: 1-800-424-9300, Outside USA: 001-703-527-3887 (collect calls accepted, Contract 17729) SECTION 2: Hazard identification 2.1. Classification of the substance or mixture GHS US classification Simple asphyxiant Press. Gas (Ref. Liq.) H281 2.2. Label elements GHS US labeling Hazard pictograms (GHS US) : GHS04 Signal word (GHS US) : Warning Hazard statements (GHS US) : H281 - CONTAINS REFRIGERATED GAS; MAY CAUSE CRYOGENIC BURNS OR INJURY OSHA-H01 - MAY DISPLACE OXYGEN AND CAUSE RAPID SUFFOCATION. CGA-HG03 - MAY INCREASE RESPIRATION AND HEART RATE. Precautionary statements (GHS US) : P202 - Do not handle until all safety precautions have been read and understood. P271+P403 - Use and store only outdoors or in a well-ventilated place. -

Liquid Carbon Dioxide (CO2) SDS (Safety Data Sheet) P-4573-D
Product: Carbon Dioxide, Refrigerated Liquid P-4573-D Date: December 2009 Praxair Material Safety Data Sheet 1. Chemical Product and Company Identification Product Name: Carbon dioxide, refrigerated liquid Trade Names: Liquiflow™ Liquid Carbon (MSDS No. P-4573-D) Dioxide, Medipure® Liquid Carbon Dioxide Chemical Name: Carbon dioxide Synonyms: Carbon dioxide (cryogenic liquid), LCO2, liquefied CO2 Chemical Family: Acid anhydride Product Grades: Industrial, USP Telephone: Emergencies: 1-800-645-4633* Company Name: Praxair, Inc. CHEMTREC: 1-800-424-9300* 39 Old Ridgebury Road Routine: 1-800-PRAXAIR Danbury, CT 06810-5113 * Call emergency numbers 24 hours a day only for spills, leaks, fire, exposure, or accidents involving this product. For routine information, contact your supplier, Praxair sales representative, or call 1-800-PRAXAIR (1-800-772-9247). 2. Hazards Identification EMERGENCY OVERVIEW WARNING! Cold liquid and gas under pressure. Can cause rapid suffocation. Can increase respiration and heart rate. May cause nervous system damage. May cause frostbite. May cause dizziness and drowsiness. Self-contained breathing apparatus and protective clothing may be required by rescue workers. This product is a colorless, odorless liquid that transforms to white crystalline particles when discharged from its container. The gas is slightly acidic and may be felt to have a slight, pungent odor and biting taste. OSHA REGULATORY STATUS: This material is considered hazardous by the OSHA Hazard Communications Standard (29 CFR 1910.1200). POTENTIAL HEALTH EFFECTS: Effects of a Single (Acute) Overexposure Inhalation. Carbon dioxide gas is an asphyxiant with effects due to lack of oxygen. It is also physiologically active, affecting circulation and breathing. -

Ocean Storage
277 6 Ocean storage Coordinating Lead Authors Ken Caldeira (United States), Makoto Akai (Japan) Lead Authors Peter Brewer (United States), Baixin Chen (China), Peter Haugan (Norway), Toru Iwama (Japan), Paul Johnston (United Kingdom), Haroon Kheshgi (United States), Qingquan Li (China), Takashi Ohsumi (Japan), Hans Pörtner (Germany), Chris Sabine (United States), Yoshihisa Shirayama (Japan), Jolyon Thomson (United Kingdom) Contributing Authors Jim Barry (United States), Lara Hansen (United States) Review Editors Brad De Young (Canada), Fortunat Joos (Switzerland) 278 IPCC Special Report on Carbon dioxide Capture and Storage Contents EXECUTIVE SUMMARY 279 6.7 Environmental impacts, risks, and risk management 298 6.1 Introduction and background 279 6.7.1 Introduction to biological impacts and risk 298 6.1.1 Intentional storage of CO2 in the ocean 279 6.7.2 Physiological effects of CO2 301 6.1.2 Relevant background in physical and chemical 6.7.3 From physiological mechanisms to ecosystems 305 oceanography 281 6.7.4 Biological consequences for water column release scenarios 306 6.2 Approaches to release CO2 into the ocean 282 6.7.5 Biological consequences associated with CO2 6.2.1 Approaches to releasing CO2 that has been captured, lakes 307 compressed, and transported into the ocean 282 6.7.6 Contaminants in CO2 streams 307 6.2.2 CO2 storage by dissolution of carbonate minerals 290 6.7.7 Risk management 307 6.2.3 Other ocean storage approaches 291 6.7.8 Social aspects; public and stakeholder perception 307 6.3 Capacity and fractions retained -

Carbon Dioxide
Safetygram 18 Carbon dioxide Carbon dioxide is nonflammable, colorless, and odorless in the gaseous and liquid states. Carbon dioxide is a minor but important constituent of the atmosphere, averaging about 0.036% or 360 ppm by volume. It is also a normal end-prod- uct of human and animal metabolism. Dry carbon dioxide is a relatively inert gas. In the event moisture is present in high concentrations, carbonic acid may be formed and materials resistant to this acid should be used. High flow rates or rapid depressurization of a system can cause temperatures approaching the sublimation point (–109.3°F [–78.5°C]) to be attained within the system. Carbon dioxide will convert directly from a liquid to a solid if the liquid is depressurized below 76 psia (61 psig). The use of ma- terials which become brittle at low temperatures should be avoided in applications where temperatures less than –20°F (–29°C) are expected. Vessels and piping used in carbon dioxide service should be designed to the American Society of Mechanical Engineers (ASME) or Department of Transportation (DOT) codes for the pressures and temperatures involved. Physical properties are listed in Table 1. Carbon dioxide in the gaseous state is colorless and odorless and not easily detectable. Gaseous carbon dioxide is 1.5 times denser than air and therefore is found in greater concentrations at low levels. Ventilation systems should be designed to exhaust from the lowest levels and allow make-up air to enter at a higher level. Manufacture Carbon dioxide is produced as a crude by-product of a number of manufactur- ing processes. -

In Vitro Antibacterial Activity of 34 Plant Essential Oils Against Alternaria Alternata
E3S Web o f Conferences 136, 06006 (2019) https://doi.org/10.1051/e3sconf/20191360 6006 ICBTE 2019 In vitro antibacterial activity of 34 plant essential oils against Alternaria alternata Qiyu Lu1, Ji Liu2, Caihong Tu2, Juan Li3, Chunlong Lei3, Qiliang Guo2, Zhengzhou Zhang2, Wen Qin1* 1College of Food Science and Technology, Sichuan Agricultural University, Ya'an, Sichuan, 625014, China 2Institute of Food Science and Technology, Chengdu Academy of Agricultural and Forestry Science, Chengdu, Sichuan, 611130, China 3 Institute of Animal Science, Chengdu Academy of Agricultural and Forestry Science, Chengdu, Sichuan, 611130, China Abstract. To determine the antibacterial effect of 34 plant essential oils on Alternaria alternata, 34 plant essential oils such as asarum essential oil, garlic essential oil, and mustard essential oil are used as inhibition agents to isolate A. alternata from citrus as indicator bacteria, through the bacteriostasis test and drug susceptibility test, the types of essential oils with the best inhibitory effect were screened and their concentration was determined. The results showed that the best inhibition effect was mustard essential oil with a minimum inhibitory concentration of 250 μl/L and a minimum bactericidal concentration of 250 μl/L. Followed by the Litsea cubeba essential oil and basil oil, the minimum inhibitory concentration is 500 μl/L. 1 Introduction quality and safety. A. alternata is the main fungus causing post-harvest disease of Pitaya, and the Citrus is a general term for orange, mandarin, orange, antibacterial effect of using cinnamon oil and clove oil is kumquat, pomelo, and medlar. Citrus is the world's good [10]. Clove oil can effectively inhibit the growth of largest fruit, rich in sugar, organic acids, mineral A. -

Herbs, Spices and Essential Oils
Printed in Austria V.05-91153—March 2006—300 Herbs, spices and essential oils Post-harvest operations in developing countries UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Telephone: (+43-1) 26026-0, Fax: (+43-1) 26926-69 UNITED NATIONS FOOD AND AGRICULTURE E-mail: [email protected], Internet: http://www.unido.org INDUSTRIAL DEVELOPMENT ORGANIZATION OF THE ORGANIZATION UNITED NATIONS © UNIDO and FAO 2005 — First published 2005 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to: - the Director, Agro-Industries and Sectoral Support Branch, UNIDO, Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria or by e-mail to [email protected] - the Chief, Publishing Management Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the United Nations Industrial Development Organization or of the Food and Agriculture Organization of the United Nations concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Carbon Dioxide (Co2)
CARBON DIOXIDE (CO2) Carbon dioxide (CO2) is colorless. At low concentrations, the gas is odorless. At higher concentrations it has a sharp, acidic odor. It is slightly toxic and has a threshold limit value of 5,000 ppm. At standard temperature and pressure, the density of carbon dioxide is around 1.98 kg/m3, about 1.5 times that of air. At atmospheric pressure and a temperature of −78.51 °C (−109.32 °F), carbon dioxide changes directly from a solid phase to a gaseous phase through sublimation, or from gaseous to solid through deposition. Liquid carbon dioxide forms only at pressures above 5.1 atm; the triple point of carbon dioxide is about 518 kPa at −56.6 °C. The boiling point of the liquid is -70°F to +88°F, depending on pressure. When vaporized at 60°F, the expansion ratio is 535:1. CO2 exists as a gas or solid below 60 psig. In liquid form, carbon dioxide weighs 4.9 pounds per gallon. In gaseous form it is slightly heavier than air. CO2 is mainly produced as an unrecovered side product of four technologies: combustion of fossil fuels, production of hydrogen by steam reforming, ammonia synthesis, and fermentation. It can be obtained by or from air distillation, however, this method is inefficient. Acute carbon dioxide physiological effect is hypercapnia or asphyxiation. Inhalation of CO2 can be tolerated at three percent inspired concentrations for at least one month and four percent inspired concentrations for over a week. Increased concentrations increase the breathing rate – e.g., 5% CO2 in air increases breathing rate by 300%. -
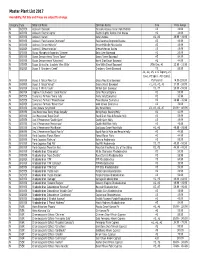
Master Plant List 2017.Xlsx
Master Plant List 2017 Availability, Pot Size and Prices are subject to change. Category Type Botanical Name Common Name Size Price Range N BREVER Azalea X 'Cascade' Cascade Azalea (Glenn Dale Hybrid) #3 49.99 N BREVER Azalea X 'Electric Lights' Electric Lights Double Pink Azalea #2 44.99 N BREVER Azalea X 'Karen' Karen Azalea #2, #3 39.99 - 49.99 N BREVER Azalea X 'Poukhanense Improved' Poukhanense Improved Azalea #3 49.99 N BREVER Azalea X 'Renee Michelle' Renee Michelle Pink Azalea #3 49.99 N BREVER Azalea X 'Stewartstonian' Stewartstonian Azalea #3 49.99 N BREVER Buxus Microphylla Japonica "Gregem' Baby Gem Boxwood #2 29.99 N BREVER Buxus Sempervirens 'Green Tower' Green Tower Boxwood #5 64.99 N BREVER Buxus Sempervirens 'Katerberg' North Star Dwarf Boxwood #2 44.99 N BREVER Buxus Sinica Var. Insularis 'Wee Willie' Wee Willie Dwarf Boxwood Little One, #1 13.99 - 21.99 N BREVER Buxus X 'Cranberry Creek' Cranberry Creek Boxwood #3 89.99 #1, #2, #5, #15 Topiary, #5 Cone, #5 Spiral, #10 Spiral, N BREVER Buxus X 'Green Mountain' Green Mountain Boxwood #5 Pyramid 14.99-299.99 N BREVER Buxus X 'Green Velvet' Green Velvet Boxwood #1, #2, #3, #5 17.99 - 59.99 N BREVER Buxus X 'Winter Gem' Winter Gem Boxwood #5, #7 59.99 - 99.99 N BREVER Daphne X Burkwoodii 'Carol Mackie' Carol Mackie Daphne #2 59.99 N BREVER Euonymus Fortunei 'Ivory Jade' Ivory Jade Euonymus #2 35.99 N BREVER Euonymus Fortunei 'Moonshadow' Moonshadow Euonymus #2 29.99 - 35.99 N BREVER Euonymus Fortunei 'Rosemrtwo' Gold Splash Euonymus #2 39.99 N BREVER Ilex Crenata 'Sky Pencil' -

Carbon Dioxide
www.scifun.org CARBON DIOXIDE Atmospheric Carbon Dioxide Carbon dioxide, CO2, is one of the gases in our atmosphere. Both natural processes and human activities contribute to its presence at a present concentration of about 0.040% [406 parts per million (ppm) on January 7, 2017], uniformly distributed over the Earth. Commercially, CO2 finds uses as a refrigerant (dry ice is solid CO2), in beverage carbonation, and in fire extinguishers. Because the concentration of CO2 in the atmosphere is low, no practical process has yet been developed to obtain the gas by extracting it from air. Most commercial CO2 is recovered as a by-product of other processes, such as the production of ethanol by fermentation and the manufacture of ammonia. Some CO2 is obtained from the combustion of coke or other carbon-containing fuels. C(coke) + O2(g) → CO2(g) Carbon dioxide is released into our atmosphere when carbon-containing fossil fuels such as oil, natural gas, and coal are burned in air. As a result of the tremendous world-wide consumption of such fossil fuels, the amount of CO2 in the atmosphere has increased over the past two centuries, now rising at a rate of about 2-3 ppm per year, Figure 1. Figure 1. This is the Keeling curve, named for the scientist, Charles Keeling, who began systematic monitoring of the atmospheric CO2 concentration at a site on the top of the Mauna Loa volcano in Hawaii in 1957. These are the usual measurements quoted in news reports and articles on atmospheric CO2 levels. Other stations around the world are now also monitoring atmospheric CO2, but Keeling’s are the longest continuous measurements. -

Active Metabolites of the Genus Piper Against Aedes Aegypti: Natural Alternative Sources for Dengue Vector Control
Univ. Sci. 2015, Vol. 20 (1): 61-82 doi: 10.11144/Javeriana.SC20-1.amgp Freely available on line REVIEW ARTICLE Active metabolites of the genus Piper against Aedes aegypti: natural alternative sources for dengue vector control André M. Marques1 , Maria Auxiliadora C. Kaplan Abstract The mosquito, Aedes aegypti, is the principal vector of the viruses responsible for dengue and dengue hemorrhagic fevers. The mosquito is widespread throughout tropical and sub-tropical regions; its prevalence makes dengue one of the most important mosquito-borne viral diseases in the world occurring annually in more than 100 endemic countries. Because blood is essential to their development cycle, the Aedes species maintains a close association with humans and their dwellings. Fittingly, the most widely adopted strategy to decrease the incidence of these diseases is the control of the mosquito larvae population. The emergence of insecticide-resistant mosquitoes has amplified the interest in finding natural products effective againstAedes aegypti adults, as well as larvae. Plant- derived compounds have played an important role in the discovery of new active entities for vector management as they are safer and have lower toxicity to humans in comparison to conventional insecticides. This review assesses a naturally occurring plant matrix and pure compounds of the Piper species, which have been shown to be active against Aedes aegypti. Keywords: dengue; Aedes aegypti; Piper; Piperaceae; larvicidal metabolites; tropical diseases. Introduction Edited by Alberto Acosta Dengue: an emerging disease in the world 1. Instituto de Pesquisas de Produtos Naturais (IPPN), Centro de Dengue is transmitted to humans by the Aedes aegypti Ciências da Saúde, Bloco H - 1º andar, Universidade Federal do Rio de Janeiro.