Making Waves the Effects of Boat-Wash on Macrobenthic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
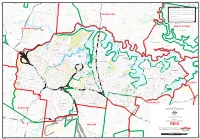
Map of the Division of Reid
REID S wa in Campbell es C Park re B e EA k 151°10'E C M ON HART OO D LA S AN M Lowanna F AR L O IE H D S VE O S OOLAROO Park Coolaroo P L A L C D S T E D N E T I R Park Chatswood FARRAN E ST D F T WILLOUGHBY Y E T Athletic S V R A D Park HA E W 151°9'E MPL K MOWBRAY RD W Y R LANE COVEMOWBRAY L AL RD W W T MA Lane RD DA H G S DA DRA LA T T LA N MINDARIE ST G D CA Cove N S NORTH OR R D MOW R E A BRAY O E C T ON T R P O L CR N P V S ES S R National I E L eek A NG T I r UNN C T L T RD rk N T A E A ba A y S M L W MURRAY S Park g O P D rin P R E St Upper A 151°8'E C A Batten T N E Lower ROSLYN CE I R I ST ON E T Stringybark C D Y S A G Y V re P Stringybark MURRA L A T ek MER SOURCESCreek Lower Stringybark V A N C R W O E E Creek S RD OX A R RT H D Reserve Creek E THIRD AVE O S D AT Stringybark K M T R CA Reserve Stringybark O T L RES O RF a A C Creek Reserve T K N N S E N T AVE r NB Reserve S E OR E A T l R N A P D T HA i ORIO C TON LL S n HNS RI RD N A P W OV JO Reserve H K E R S F Y W E T G E L s Batten T M T L O T L S I O A O E RR S N IZ N H L RD O S N A G W TU E I M AB T K G H Magdala RE E R E Creek N L P B AR D 151°7'E N A E D S R E A KL S R This map has been compiled Nby Spatial Vision from data supplied by the AustralianAND H L CLE T E L ST Park E A NUNDA U H L ER O D O AD S T D D S Tantallon O R Reserve P RD Z A S O Pryor D G T Y A W A I S G Y VE R R N N T T O P I V D T S R K A T Electoral Commission, Geoscience Australia, DepartmentL ofT the Environment, Water, HANCOTT ST R U R O K A T T IN Park E S D E S D F S S E B M D Park i E W V R P Wallumatta tt PAG S G R N U CL G L y E T ARA A U s ER LAND K C N F S Heritage andLD the Arts andB PitneyRD Bowes Business Insight. -

Dragonfly Survey Report
Moorebank Precinct West Threatened Dragonfly Species Survey Plan Report Part 4, Division 4.1, State Significant Development September 2016 TACTICAL GROUP MOOREBANK PRECINCT WEST Threatened Dragonfly Species Survey Plan Report Author Adam Costenoble Checker Kate Carroll Approver Ketan Patel Report No 001 Date 26/09/2016 Revision Text Final This report has been prepared for Tactical Group in accordance with the terms and conditions of appointment for AA009335 dated July 2016. Arcadis Australia Pacific Pty Limited (ABN 76 104 485 289) cannot accept any responsibility for any use of or reliance on the contents of this report by any third party. REVISIONS Prepared Approved Revision Date Description by by Adam 001 26/09/16 For submission to DPI Fisheries Ketan Patel Costenoble V i CONTENTS EXECUTIVE SUMMARY ............................................................................................................ 1 1 INTRODUCTION ...................................................................................................................... 2 1.1 Project Overview ................................................................................................................. 2 1.2 Purpose of this report ......................................................................................................... 3 1.3 Aims of this Report ............................................................................................................. 3 1.4 Consultation ....................................................................................................................... -

Cape York Peninsula Parks and Reserves Visitor Guide
Parks and reserves Visitor guide Featuring Annan River (Yuku Baja-Muliku) National Park and Resources Reserve Black Mountain National Park Cape Melville National Park Endeavour River National Park Kutini-Payamu (Iron Range) National Park (CYPAL) Heathlands Resources Reserve Jardine River National Park Keatings Lagoon Conservation Park Mount Cook National Park Oyala Thumotang National Park (CYPAL) Rinyirru (Lakefield) National Park (CYPAL) Great state. Great opportunity. Cape York Peninsula parks and reserves Thursday Possession Island National Park Island Pajinka Bamaga Jardine River Resources Reserve Denham Group National Park Jardine River Eliot Creek Jardine River National Park Eliot Falls Heathlands Resources Reserve Captain Billy Landing Raine Island National Park (Scientific) Saunders Islands Legend National Park National park Sir Charles Hardy Group National Park Mapoon Resources reserve Piper Islands National Park (CYPAL) Wen Olive River loc Conservation park k River Wuthara Island National Park (CYPAL) Kutini-Payamu Mitirinchi Island National Park (CYPAL) Water Moreton (Iron Range) Telegraph Station National Park Chilli Beach Waterway Mission River Weipa (CYPAL) Ma’alpiku Island National Park (CYPAL) Napranum Sealed road Lockhart Lockhart River Unsealed road Scale 0 50 100 km Aurukun Archer River Oyala Thumotang Sandbanks National Park Roadhouse National Park (CYPAL) A r ch KULLA (McIlwraith Range) National Park (CYPAL) er River C o e KULLA (McIlwraith Range) Resources Reserve n River Claremont Isles National Park Coen Marpa -

Download Our 2021 Lizard Island Experiences Brochure
YOUR ISLAND EXPERIENCES CUSTOMISED Experience Lizard Island on the reef, on land or in the tranquillity of Note charges apply for certain activities. All tours and activities the spa. are subject to change due to weather or tidal conditions. All ITINERARY Lizard Island offers a wide range of activities that provide opportunities timings are approximate and minimum and maximum tour to fully immerse yourself in the natural attractions of the island and numbers may apply. surrounding Great Barrier Reef. Our experienced and enthusiastic guides are adept at personalising your experience to your individual tastes, creating memories that will last a lifetime. Allow us to tailor you a customised itinerary to ensure you sample the best of Lizard Island and the Great Barrier Reef while still leaving time to take in the serenity of your room. Guests are encouraged to pre-book either their full itinerary or individual excursions prior to arrival. 2 lizardisland.com.au 3 QUICK FACTS LOCATION CLIMATE AND WATER Lizard Island is located 240km north of Cairns and TEMPERATURE 27km off the coast of Tropical North Queensland. It Average year-round temperature of 27° C (80° F) is a National Park covering 1,013ha with magnificent Winter water temperature: 22-24° C (72-76° F) sandy beaches. Summer water temperature: 27-30° C (81-86° F) THINGS YOU CAN SEE Full length wetsuits, that are available in all sizes on Island, are recommended between May and Marine Life October. Amongst the estimated 1,500 different fish species, the favourites to look for include the Potato Cod MARINE STINGERS and Giant Grouper, schooling trevallies, giant sweetlips, reef sharks, olive sea snakes, turtles, rays, Jellyfish may be present in the waters during cuttlefish, anemone fish and giant clams. -

Estuary Surveillance for QX Disease
Estuary surveillance Student task sheet for QX disease The following tables show data collected Estuary Surveillance 2002: during estuary surveillance from 2001– During the 2002 sampling period a total of 2004 for New South Wales and 5250 oysters were received and processed Queensland. N is the number of oysters from 18 NSW estuaries and three tested in a random sample of the oyster Queensland zones using tissue imprints. population. Dr Adlard used two methods of disease detection in surveillance — tissue imprint and PCR. Table 2A: Tissue imprints used to detect the QX disease parasite Estuary Surveillance 2001: 2002 Survey results Table 1: Tissue imprint results for 2001 N 2001 Survey Results Estuary N infected % N Northern Moreton Bay 250 0 0 Estuary N infected % Central Moreton Bay 250 0 0 Tweed River 316 0 0 Southern Moreton Bay 250 2 0.8 Brunswick River 320 0 0 Tweed River 250 0 0 Richmond River 248 0 0 Brunswick River 250 0 0 Clarence River 330 5 1.52 Richmond River 250 102 40.8 Wooli River 294 0 0 Clarence River 250 55 22 Kalang /Bellinger 295 0 0 Wooli River 250 0 0 Rivers Kalang /Bellingen Rivers 250 0 0 Macleay River 261 0 0 Macleay River 250 0 0 Hastings River 330 0 0 Hastings River 250 0 0 Manning River 286 0 0 Manning River 250 0 0 Wallis Lakes 271 0 0 Wallis Lakes 250 0 0 Port Stephens 263 0 0 Port Stephens 250 0 0 Hawkesbury River 323 0 0 Hawkesbury River 250 0 0 Georges River 260 123 47.31 Georges River 250 40 16 Shoalhaven/ 255 0 0 Crookhaven Shoalhaven/Crookhaven 250 0 0 Bateman's Bay 300 0 0 Bateman's Bay 250 0 0 Tuross Lake 304 0 0 Tuross Lake 250 0 0 Narooma 300 0 0 Narooma 250 0 0 Merimbula 250 0 0 Merimbula 250 0 0 © Queensland Museum 2006 Table 2B: PCR results from 2002 on Estuary Surveillance 2003: oysters which had tested negative to QX During 2003 a total of 4450 oysters were disease parasite using tissue imprints received and processed from 22 NSW estuaries and three Queensland zones. -

Population Monitoring of Glenelg Spiny Crayfish (Euastacus Bispinosus ) in Rising-Spring Habitats of Lower South East, South Australia
Population monitoring of Glenelg Spiny Crayfish (Euastacus bispinosus ) in rising-spring habitats of lower south east, South Australia Nick Whiterod and Michael Hammer Aquasave Consultants, Adelaide May 2012 Report to Friends of Mt Gambier Area Parks (Friends of Parks Inc.) Citation Whiterod, N. and Hammer, M. (2012). Population monitoring of Glenelg Spiny Crayfish (Euastacus bispinosus ) in rising-spring habitats of lower south east, South Australia. Report to Friends of Mt Gambier Area Parks (Friends of Parks Inc.). Aquasave Consultants, Adelaide. p. 27. Correspondence in relation to this report contact Dr Nick Whiterod Aquasave Consultants Tel: +61 409 023 771 Email: [email protected] Photographs © N. Whiterod, O. Sweeney, D. Mossop and M. Hammer Cover (clockwise from top): underwater view of aquatic vegetation in the Ewens Pond system; female Glenelg Spiny Crayfish with eggs (in berry); and Glenelg Spiny Crayfish amongst aquatic vegetation. Disclaimer This report was commissioned by Friends of Mt Gambier Area Parks (Friends of Parks Inc.). It was based on the best information available at the time and while every effort has been made to ensure quality, no warranty express or implied is provided for any errors or omissions, nor in the event of its use for any other purposes or by any other parties. ii Table of Contents 1.0 Introduction ........................................................................................................................... 1 2.0 Methods .................................................................................................................................. -

Congolli (Pseudaphritis Urvillii) and Australian Salmon (Arripis Truttaceus and A
Inland Waters and Catchment Ecology Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong George Giatas and Qifeng Ye SARDI Publication No. F2015/000479-1 SARDI Research Report Series No. 858 SARDI Aquatics Sciences PO Box 120 Henley Beach SA 5022 September 2015 Giatas and Ye (2015) Diet of three fish species in the Coorong Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong George Giatas and Qifeng Ye SARDI Publication No. F2015/000479-1 SARDI Research Report Series No. 858 September 2015 II Giatas and Ye (2015) Diet of three fish species in the Coorong This publication may be cited as: Giatas, G.C. and Ye, Q. (2015). Diet and trophic characteristics of mulloway (Argyrosomus japonicus), congolli (Pseudaphritis urvillii) and Australian salmon (Arripis truttaceus and A. trutta) in the Coorong. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. SARDI Publication No. F2015/000479-1. SARDI Research Report Series No. 858. 81pp. South Australian Research and Development Institute SARDI Aquatic Sciences 2 Hamra Avenue West Beach SA 5024 Telephone: (08) 8207 5400 Facsimile: (08) 8207 5406 http://www.pir.sa.gov.au/research DISCLAIMER The authors warrant that they have taken all reasonable care in producing this report. The report has been through the SARDI internal review process, and has been formally approved for release by the Research Chief, Aquatic Sciences. Although all reasonable efforts have been made to ensure quality, SARDI does not warrant that the information in this report is free from errors or omissions. -

Club Veedub Sydney. December 2014
NQ629.2220994/5 Club VeeDub Sydney. www.clubvw.org.au Merry Christmas to all our members! December 2014 IN THIS BUMPER CHRISTMAS ISSUE: Eastern Creek Marshals Marques in the Park Boris’ Picnic Day Snowy Mountains 1000 Pie In the Sky Run Eat St Parramatta 1956 Chicago Run Plus lots more... Club VeeDub Sydney. www.clubvw.org.au A member of the NSW Council of Motor Clubs. Also affiliated with CAMS. ZEITSCHRIFT - December 2014 - Page 1 Club VeeDub Sydney. www.clubvw.org.au Club VeeDub Sydney Club VeeDub membership. Membership of Club VeeDub Sydney is open to all Committee 2014-15. Volkswagen owners. The cost is $45 for 12 months. President: Steve Carter 0490 020 338 [email protected] Monthly meetings. Monthly Club VeeDub meetings are held at the Vice President: Craig Adams 0404 184 893 Greyhound Social Club Ltd., 140 Rookwood Rd, Yagoona, on [email protected] the third Thursday of each month, from 7:30 pm. All our members, friends and visitors are most welcome. Secretary and: Norm Elias 0421 303 544 Membership: [email protected] Correspondence. Assist. Secretary: David Birchall (02) 9534 4825 Club VeeDub Sydney [email protected] PO Box 1340 Camden NSW 2570 Treasurer: Martin Fox 0411 331 121 [email protected] Our magazine. Editor: Phil Matthews 0412 786 335 Zeitschrift (German for ‘magazine’) is published monthly [email protected] by Club VeeDub Sydney Inc. Members with email details receive Assistant Editor: Lily Matthews Zeitschrift as a full-colour PDF from our website. We welcome all letters and contributions of general VW interest. -

A New Freshwater Crab of the Family Hymenosomatidae Macleay, 1838
A new freshwater crab of the family Hymenosomatidae MacLeay, 1838 (Crustacea, Decapoda, Brachyura) and an updated review of the hymenosomatid fauna of New Caledonia Danièle Guinot, Valentin de Mazancourt To cite this version: Danièle Guinot, Valentin de Mazancourt. A new freshwater crab of the family Hymenosomatidae MacLeay, 1838 (Crustacea, Decapoda, Brachyura) and an updated review of the hymenosomatid fauna of New Caledonia. European Journal of Taxonomy, Consortium of European Natural History Museums, 2020, pp.1-29. 10.5852/ejt.2020.671. hal-02885460 HAL Id: hal-02885460 https://hal.archives-ouvertes.fr/hal-02885460 Submitted on 30 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. European Journal of Taxonomy 671: 1–29 ISSN 2118-9773 https://doi.org/10.5852/ejt.2020.671 www.europeanjournaloftaxonomy.eu 2020 · Guinot D. & Mazancourt V. de This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:9EF19154-D2FE-4009-985C-1EB62CC9ACB0 A new freshwater crab of the family Hymenosomatidae MacLeay, 1838 from New Caledonia (Crustacea, Decapoda, Brachyura) and an updated review of the hymenosomatid fauna of New Caledonia Danièle GUINOT 1,* & Valentin de MAZANCOURt 2 1 ISYEB (CNRS, MNHN, EPHE, Sorbonne Université), Institut Systématique Évolution Biodiversité, Muséum national d’histoire naturelle, case postale 53, 57 rue Cuvier, 75231 Paris cedex 05, France. -

Sydney Harbour Superyacht Guidelines
Sydney Harbour superyacht guidelines Guidelines for Masters operating Superyachts on Sydney Harbour Contents Executive Summary 1 Qualifications and registration 9 Port procedures 2 Boat licences and certificates of competency 9 Directions for navigation 2 Registration of vessels 9 Directions and regulations to be observed 2 Protected animals 10 Required charts 2 Approach distances 10 Port services 2 Speed 10 Pilotage requirements 2 Approach directions 10 Wind and weather 3 Action if a marine mammal approaches 11 Port Authority of NSW Vessel Traffic Service 3 Communications 11 Pilot boarding place 3 VHF channels 11 Sydney Harbour – general considerations 3 Important contact details 11 General 3 Useful websites 12 Speed limits 3 Photographs 13 Speed restricted areas 4 Anzac Bridge 13 Conduct within Sydney Harbour 7 Rozelle Bay Superyacht Marina 13 Prohibited areas for general navigation 7 Campbells Cove 14 General 7 Sydney Cove – Circular Quay 15 Restricted access areas 7 Fort Denison 15 Collision or incident reports 8 Garden Island Naval Base 15 Berthing at commercial wharves 8 Walsh Bay 16 Pollution, nuisance or danger 8 Sydney Harbour Bridge 17 Marine Pollution Act 1987 8 Jones Bay Wharf, Pyrmont 17 Pump-out facilities 8 Kirribilli Point 17 Garbage 9 Anzac Bridge 18 Causing of nuisance or danger 9 Glebe Island Bridge 18 Farm Cove 18 Wind frequency analyses 19 FRONT COVER PHOTO: ANDREA FRANCOLINI Executive Summary Welcome to Sydney. The aim of these guidelines is to assist superyacht masters Superyachts are free to enter and move around with their preparations for a visit to Sydney Harbour and to Sydney Harbour subject to compliance with the provide a reference document during the visit. -

The March 1978 Flood on the Hawkesbury and Nepean
... I'., The March, 1978 flood on the Hawkesbury and Nepean River between Penrith and Pitt Town S. J. Riley School of Earth Sciences, Macquarie University, - North Ryde. N. S.W. 2113 .. ,.. ... .. ... ..... .. - ~ . .. '~,i';~;: '~ It'i _:"to "\f',. .,.,. ~ '.! . I .... I ,', ; I I ' }, I , I , I The March, 1978 flood on the I Hawkesbury and Nepean River I .. between PenDth and Pitt Town I I I I S.J. Riley 1 f I :''',i I I School of Earth Sciences, I Macquarie University, ·1 North Ryde. N.S.W. 2113 I I',.. , ··1 " " ., ~: ". , r-~.I··_'~ __'_'. ~ . '.," '. '..a.w-.,'",' --~,~"; l .' . - l~' _I,:.{·_ .. -1- Introduction As a result of three days of heavy rainfall over the Hawkesbury c:ltchment in March, 1978 floods occurred on all the streams in the Hawkesbury system. These floods caused considerable property damage and resulted in morphological changes to the channels and floodplains 1 of, the Hawkesbury system. This paper describes the flodd in the Hawkesbury-Nepean system in the reach'extending from Penrith to Pitt Town •. Storm Pattern An intense low pressure cell developed over the Coral Sea on the 16th March, 1978. This low pressure system travelled southeast towards the Queensland coast and gained in intensity (Fig.l). On the 18th March it,appeared that the cell would move eastwards away from Australia. However, the system reversed its direction of travel and moved inland. Resultant wind systems brought warm moist air from ,the east onto the .. " coast of New South Wal,es. Consequently, heavy rainfall$ occurred from f I .. the 18th to 24th March over the whole of eastern New South Wales. -

Reducing the Impact of Weirs on Aquatic Habitat
REDUCING THE IMPACT OF WEIRS ON AQUATIC HABITAT NSW DETAILED WEIR REVIEW REPORT TO THE NEW SOUTH WALES ENVIRONMENTAL TRUST SYDNEY METROPOLITAN CMA REGION Published by NSW Department of Primary Industries. © State of New South Wales 2006. This publication is copyright. You may download, display, print and reproduce this material in an unaltered form only (retaining this notice) for your personal use or for non-commercial use within your organisation provided due credit is given to the author and publisher. To copy, adapt, publish, distribute or commercialise any of this publication you will need to seek permission from the Manager Publishing, NSW Department of Primary Industries, Orange, NSW. DISCLAIMER The information contained in this publication is based on knowledge and understanding at the time of writing (July 2006). However, because of advances in knowledge, users are reminded of the need to ensure that information upon which they rely is up to date and to check the currency of the information with the appropriate officer of NSW Department of Primary Industries or the user‘s independent adviser. This report should be cited as: NSW Department of Primary Industries (2006). Reducing the Impact of Weirs on Aquatic Habitat - New South Wales Detailed Weir Review. Sydney Metropolitan CMA region. Report to the New South Wales Environmental Trust. NSW Department of Primary Industries, Flemington, NSW. ISBN: 0 7347 1753 9 (New South Wales Detailed Weir Review) ISBN: 978 0 7347 1833 4 (Sydney Metropolitan CMA region) Cover photos: Cob-o-corn Weir, Cob-o-corn Creek, Northern Rivers CMA (upper left); Stroud Weir, Karuah River, Hunter/Central Rivers CMA (upper right); Mollee Weir, Namoi River, Namoi CMA (lower left); and Hartwood Weir, Billabong Creek, Murray CMA (lower right).