Management of Malolactic Fermentation to Enhance Red Wine Color and Reduce the Risk of Brettanomyces Spoilage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shiraz – the New Kid on the Block Come and See What the Fuss Is All About Wednesday, October 14 at Toronto Lawn Tennis Club
Eleanor Cosman, President South African Wine Society Box 37085, 6478 Yonge St Willowdale ON M2M 4J0 905-762-9161 www.southafricanwinesociety.ca [email protected] Shiraz – the new kid on the block Come and see what the fuss is all about Wednesday, October 14 at Toronto Lawn Tennis Club South Africa has three hundred and Today, it is one of the prominent acres). In South Africa, the varietal is fifty years of wine history with the varietals used both in single varietal predominately known as shiraz, but first wines being produced by Dutch wines as well as being blended in the designation syrah is used for settlers. Originally using the local Rhône-style wines. These are big-fruit "Rhône-style" wines. Some see this vines and finding the climate and wines with chocolate, smoke, and varietal as the "great hope" for South terrain conducive to making wine, spice. African wines. The shiraz wine they brought in European vines and regions are mostly in the Western It is emerging as the Cape’s leading began planting. Cape near Cape Town. red varietal, knocking the various Shiraz was taken by French Bordeaux grapes from their perch with Come and learn what the fuss is all Hugeonots to South Africa and from increasing regularity. It is currently about. Not only will you have the there to Australia in the 17th Century. the third most planted red grape after opportunity to taste a selection pinotage and cabernet sauvignon. The assembled by cellar master Jon The production of shiraz has taken off future looks promising, although it’s Whitteker, you will hear the in climates and within economies that true that some producers have yet to comments of renowned wine favour mass production. -

Mercaptans and Other Volatile Sulfur Compounds in Wine
Mercaptans and other volatile sulfur compounds in wine © Jamie Goode 2006 www.wineanorak.com An article on volatile sulfur compounds in wine isn’t likely to turn many heads. In truth, it’s the sort of topic that gets filed away as ‘worthy but dull’, and only ever gets read by people who are swotting up for their diploma or MW, and then gets promptly forgotten. Aware of this, I’m willing to strike a deal with my readers. If I try my best to cover this otherwise fiercely dull and technical wine science piece in a manner that is both readable and doesn’t require any specialist science knowledge, will you try to venture below the first paragraph? Volatile sulfur compounds, and specifically mercaptans, are a hot topic in wine at the moment, so it’s worth learning a bit about them. These are the compounds largely responsible for the olfactory defect known as ‘reduction’. And there’s a lot of ignorance and misinformation appearing about them, even in print. My aim in this piece is to present a step-by-step guide to these molecules in wine, bringing in some of the latest research and providing an accessible introduction to the subject without skimping on the meaty bits. There are around 100 volatile sulfur compounds that have been identified in wine, but only a few are significant enough to be included in our story here. What do you need to know about them? First and foremost, they are smelly. Thus even at low concentrations they can have a sensory impact on the wine. -
Wine Spectator
TASTING HIGHLIGHTS 9 West Coast Red Blends for Sweater Weather Cabernet Sauvignon, Merlot, Syrah and more newly reviewed wines from California and Washington Some of Limerick Lane's vines date to the !rst plantings in 1910. (Richard Knapp) By Augustus Weed Oct 7, 2019 Tasting Highlights' wine reviews are fresh out of the tasting room, o!ering a sneak peek of our editors' most recent scores and notes to WineSpectator.com members. Red blends are red hot these days, with winemakers across the West Coast making both oddball and traditional-style blends from a variety of di"erent grapes, such as Cabernet Sauvignon, Syrah and everything in between. Today's selection casts a wide net with highly rated wines from California and Washington. Topping the list is a gutsy blend from Limerick Lane [https://www.winespectator.com/wine/search/submitted/Y/search_by/exact/text_search_#ag/winery/winery/Limerick+Lane] . Winemaker Chris Pittenger combined mostly Syrah with Zinfandel and Petite Sirah from the winery's 30-acre estate vineyard in the northeast corner of the Russian River Valley appellation. Alexana [https://www.winespectator.com/wine/search/submitted/Y/search_by/exact/text_search_#ag/winery/winery/Alexana] winemaker Bryan Weil looked farther north to the Columbia Valley in Washington for the supple Gran Rouge. It's a Southern Rhône–inspired blend of Grenache, Syrah and Mourvèdre that shows how well these grapes complement each other. Eric Kent [https://www.winespectator.com/wine/search/submitted/Y/search_by/exact/text_search_#ag/winery/winery/Eric+Kent] made one of the best values here, using grapes from Mendocino County. -

A to Z Glossary of Wine Terms
A to Z Glossary of Wine Terms A ABV (Alcohol by Volume) - A measure of the alcohol concentration of a wine, usually expressed as a percentage. Acidic - A tart, sour, or fresh feeling in the mouth when you taste the wine. Aeration - Adding oxygen to the wine to soften tannins. Aggressive – Wine tasting term describing a wine that is either too tannic, too acidic, or a combina- tion of both. Alcohol - Ethanol; in wine it is produced via fermentation with yeast and sugar. Alcoholic – Wine tasting term indicating high alcohol. A wine with a noticeably high alcohol con- tent; perceived as a hotness in the wine. Angular – Wine tasting term used to describe young wines that display predominately sharp, bitter, or tart flavor characteristics. AOC (Appellation d’origine contrôlée) - A term in the French wine designation system which means “controlled designation of origin.” It identifies the location or region where a wine is made. Appearance - In general, the term appearance is used to describe the clarity of a wine. Appellation - A designated wine growing area governed by specific rules regarding the wine grapes grown and wine produced in the specific appellation areas. Aroma - A wine’s scent characteristics; very closely tied in with the flavors. Aromatic - Varieties of grapes that have especially noticeable aromas. Some aromatic grapes in- clude Viognier, Torrontés, Gewürztraminer, Riesling, Muscat, and Pinot Gris. Aromatized – A wine that is infused with botanicals. Vermouth is an aromatized wine. Assemblage - A method of blending wine before bottling. Astringent – A wine tasting term meaning the wine leaves the mouth feeling overwhelmingly dry. -

2021 Barossa Wine Auction Catalogue Here
In April 2021, Barossa Grape & Wine Association together with Langton’s Fine Wines, present Australia’s most prestigious regional wine auction. An integral part of the Partnering with Langton’s Barossa Vintage Festival Fine Wine Auction House, since 1965, the Barossa the Barossa Wine Auction Wine Auction has now brings you an exclusive grown to become opportunity to access Australia’s premier rare and covetable wines. regional wine auction. Provenance is assured, with wines sourced directly from the winery and winemaker’s own collections. Barossa Wine Auction 2020 2 Barossa Live Auction Page 5-11 (auction lots beginning ‘B’) Friday 16 April 2021 Tickets $50pp includes Eden 9.30 am – 12.30pm Valley Riesling and Oysters on arrival and light refreshments Chateau Tanunda throughout. Basedow Road _ Tanunda, SA www.barossavintagefestival.com.au Sydney Live Auction Page 13-19 (auction lots beginning ‘S’) Dinner Thursday 29 April 2021 Hyatt Regency Sydney, NSW Tickets to be released in early 2021 Online Auction Page 21-31 (auction lots beginning ‘W’) Opens Friday 9 April 2021 Closes Sunday 2 May 2021 At langtons.com.au Barossa Wine Auction 2020 3 Barossa Live Auction o LOT N- Winery Barossa Bottle Set of 9 Vintage MV Price Guide $7000.00 - 8000.00 B01 Quantity 1 An extremely rare, highly collectable set of ultra fine Barossa wines, each one awarded a perfect 100 points, includes: 1 x 375ml BOTTLE of Seppeltsfield 1921 100 Year Old Para Vintage Tawny (Halliday) 1 x BOTTLE of Torbreck 2016 RunRig Shiraz Viognier (Joe Czerwinski) 1 x BOTTLE of Torbreck 2012 The Laird Shiraz (Robert Parker) 1 x BOTTLE of Penfolds 2013 Bin 95 Grange (Wine Spectator) 1 x BOTTLE of Chris Ringland 2002 Shiraz (Robert Parker) 1 x BOTTLE of Greenock Creek 1998 Roennfeldt Road Cabernet (Robert Parker) 1 x BOTTLE of Greenock Creek 1998 Roennfeldt Road Shiraz (Robert Parker) 1 x BOTTLE of Henschke 2015 Hill of Grace Shiraz (Wine Spectator/ Andred Caillard MW) 1 x BOTTLE of Standish Wine Co. -

BUBBLES PINOT NOIR-CHARDONNAY, Pierre
Wines By The Glass BUBBLES PINOT NOIR-CHARDONNAY, Pierre Paillard, ‘Les Parcelles,’ Bouzy, Grand Cru, 25 Montagne de Reims, Extra Brut NV -treat yourself to this fizzy delight MACABEO-XARELLO-PARELLADA, Mestres, 'Coquet,' Gran Reserva, 14 Cava, Spain, Brut Nature 2013 -a century of winemaking prowess in every patiently aged bottle ROSÉ OF PINOT NOIR, Val de Mer, France, Brut Nature NV 15 -Piuze brings his signature vibrant acidity to this juicy berried fizz WHITE + ORANGE TOCAI FRIULANO, Mitja Sirk, Venezia Giulia, Friuli, Italy ‘18 14 -he made his first wine at 11; now he just makes one wine-- very well, we think FRIULANO-RIBOLLA GIALLA-chardonnay, Massican, ‘Annia,’ 17 Napa Valley, CA USA ‘17 -from the heart of American wine country, an homage to Northern Italy’s great whites CHENIN BLANC, Château Pierre Bise, ‘Roche aux Moines,’ 16 Savennières, Loire, France ‘15 -nerd juice for everyone! CHARDONNAY, Enfield Wine Co., 'Rorick Heritage,' 16 Sierra Foothills, CA, USA ‘18 -John Lockwood’s single vineyard dose of California sunshine RIESLING, Von Hövel, Feinherb, Saar, Mosel, Germany ‘16 11 -sugar and spice and everything nice TROUSSEAU GRIS, Jolie-Laide, ‘Fanucchi Wood Road,’ Russian River, CA, USA ‘18 15 -skin contact lends its textured, wild beauty to an intoxicating array of fruit 2 Wines By The Glass ¡VIVA ESPAÑA! -vibrant wines sprung from deeply rooted tradition and the passion of a new generation VIURA-MALVASIA-garnacha blanca, Olivier Rivière, ‘La Bastid,’ Rioja, Spain ‘16 16 HONDARRABI ZURI, Itsasmendi, ‘Bat Berri,’ Txakolina -

Malolactic Fermentation in Red Wine
Fact Sheet WINEMAKING Malolactic fermentation in red wine Introduction Malolactic fermentation (MLF) is a secondary bacterial fermentation carried out in most red wines. Oenococcus oeni, a member of the lactic acid bacteria (LAB) family, is the main bacterium responsible for conducting MLF, due to its ability to survive the harsh conditions of wine (high alcohol, low pH and low nutrients) and its production of desirable wine sensory attributes. One of the important roles of MLF is to confer microbiological stability towards further metabolism of L-malic acid. Specifically, MLF removes the L-malic acid in wine that can be a carbon source for yeast and bacterial growth, potentially leading to spoilage, spritz and unwanted flavours. MLF can also be conducted in some wines to influence wine style. MLF often occurs naturally after the completion of primary fermentation or can also be induced by inoculation with a selected bacterial strain. Since natural or ‘wild’ MLF can be unpredictable in both time of onset and impact on wine quality, malolactic starter cultures are commonly used. In Australia, almost all red wines undergo MLF and 74% are inoculated with bacterial starter cultures (Nordestgaard 2019). This fact sheet provides practical information for induction of MLF in red wine. A separate fact sheet provides equivalent information for white and sparkling base wine. These should also be read in conjunction with another fact sheet, Achieving successful malolactic fermentation, which gives further practical guidelines for MLF induction, monitoring and management. Updated September 2020 Fact Sheet WINEMAKING Key parameters for a successful MLF in red wine Composition of red wine/must The main wine compositional factors that determine the success of MLF are alcohol, pH, temperature and sulfur dioxide (SO2) concentration. -

Objective Measures of Shiraz Grape and Wine Quality in Premium Australian Vineyards
Objective measures of Shiraz grape and wine quality in premium Australian vineyards FINAL REPORT TO WINE AUSTRALIA Project Number: AWRI 1701-3.3.1 (formerly AWR1503) Principal Investigator: Dr Keren Bindon Research Organisation: The Australian Wine Research Institute Date: January 2020 1 Project title: Objective measures of Shiraz grape and wine quality in premium Australian vineyards Author: Dr Keren Bindon Date: January 2020 Address: The Australian Wine Research Institute, Wine Innovation Central Building, Hartley Grove, cnr Paratoo Rd, Urrbrae (Adelaide), SA 5064 Disclaimer/copyright statement: This document has been prepared by The Australian Wine Research Institute ("the AWRI") as part of fulfilment of obligations towards the Project Agreement AWR 1701-3.3.1 which were formerly under AWR1503and is intended to be used solely for that purpose and unless expressly provided otherwise does not constitute professional, expert or other advice. The information contained within this document ("Information") is based upon sources, experimentation and methodology which at the time of preparing this document the AWRI believed to be reasonably reliable and the AWRI takes no responsibility for ensuring the accuracy of the Information subsequent to this date. No representation, warranty or undertaking is given or made by the AWRI as to the accuracy or reliability of any opinions, conclusions, recommendations or other information contained herein except as expressly provided within this document. No person should act or fail to act on the basis -

2021 Musto Wine Grape Co. Harvest Menu 2021 Musto Wine Grape Co
2021 Musto Wine Grape Co. Harvest Menu 2021 Musto Wine Grape Co. Harvest Menu HARVEST IS ALMOST HERE! THE GRAPES ARE ABOUT 2-3 WEEKS AHEAD OF SCHEDULE AND SHOW NO SIGNS OF SLOWING DOWN. WE WILL HAVE SOME EARLY RIPENING GRAPES AND WINEMAKING JUICES ARRIVING THE WEEK AFTER LABOR DAY (SEPTEMBER 6TH). WE HAVE NEW WINEMAKING GRAPES AND INTERESTING VINEYARDS BEING ADDED TO THE MWG WINEMAKING PORTFOLIO THIS SEASON. BELOW YOU WILL SEE INFORMATION REGARDING OUR NEWEST ADDITIONS. PLEASE KEEP IN MIND THAT ALL RED GRAPE VARIETIES CAN BE PROCESSED INTO FROZEN MUST BY REQUEST/PRE-ORDER ONLY AND ALL WINE GRAPE VARIETIES CAN BE PURCHASED IN 6 GALLON FRESH JUICE PAILS FROM CALIFORNIA. HAVE YOU STARTED YOUR WINEMAKING WISH LIST YET? GIVE US A CALL AT THE OFFICE TO DISCUSS YOUR 2021 WINE! 877-812-1137 - [email protected] CHEERS! THE MUSTO CRUSH CREW 2021 Musto Wine Grape Co. Harvest Menu GRAPES: LANZA-MUSTO GRAPES: LODI, CA (SUISUN VALLEY, CA) BARBERA PETITE VERDOT ALICANTE ZINFANDEL CABERNET SAUVIGNON (VALLEY) PETITE SIRAH BARBERA OLD VINE ZINFANDEL CABERNET SAUVIGNON (169) PRIMITIVO CABERNET FRANC VALDEPNA CABERNET SAUVIGNON (15) TEMPRANILLO CABERNET SAUVIGNON ALBARINO CABERNET SAUVIGNON (KOCH) SYRAH (LIMITED) CARIGNANE BLACK MUSCAT MALBEC CHARDONNAY GRENACHE CHARDONNAY MERLOT RIESLING MALBEC FRENCH COLOMBARD MOURVEDRE SAUVIGNON BLANC MERLOT MALVASIA BIANCA SANGIOVESE (BRUNELLO CLONE) MUSCAT CANNELLI MIXED BLACK MUSCAT PETITE SIRAH PINOT GRIGIO GRAPES: METTLER RANCH PINOT NOIR RIESLING (LODI, CA) RUBY CABERNET SAUVIGNON BLANC SANGIOVESE THOMPSON SEEDLESS PINOTAGE SYRAH TEMPRANILLO VIOGNIER GRENACHE NOIR CABERNET SAUVIGNON FIANO VERMENTINO MERLOT ZINFANDEL PETITE SIRAH SANGIOVESE 2021 Musto Wine Grape Co. -
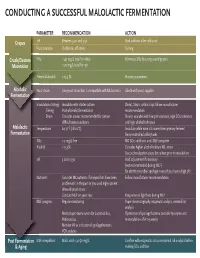
Conducting a Successful Malolactic Fermentation
CONDUCTING A SUCCESSFUL MALOLACTIC FERMENTATION PARAMETERPA RECOMMENDATION ACTION Grapes pH Between 3.20 and 3.50 Acid addition after cold soak FruitFru condition Visible rot, off odors Sorting Crush/Destem SO2SO < 40 mg/L total for white Minimize SO2 by using sound grapes Maceration < 70 mg/L total for red PotentialPPot alcohol < 13.5 % Harvest parameters Alcoholic YeastYeY a strain Use yeast strain that is compatible with ML bacteria Check with yeast supplier Fermentation InoculationIno strategy Inoculate with starter culture Direct, Step 1, or Build up: follow manufacturer Timing Post-alcoholic fermentation recommendation Strain Consider strains recommended for certain Strains available with low pH tolerance, high SO2 tolerance difficult wine conditions and high alcohol tolerance Malolactic TemperatureTem 64-71°F (18-22°C) Inoculate while wine still warm from primary ferment Fermentation Temp-controlled cellar/tanks SO2SO < 5 mg/L free NO SO2 additions until MLF complete AlcoholAlc < 13.5% Consider higher alcohol tolerant ML strain Use acclimatization steps for culture prior to inoculation pH 3.20 to 3.50 Acid adjustment if necessary (not recommended during MLF) Be alert to microbial spoilage issues if you have a high pH NNutrientsu Consider ML nutrients if vineyard lots have been Follow manufacturer recommendation problematic in the past or you used high nutrient demand yeast strain Conduct MLF on yeast lees Keep wine on light lees during MLF MMLFL progress Regular monitoring Paper chromatography, enzymatic analysis, external lab analysis Microscopic examination for Lactobacillus, If presence of spoilage bacteria consider lysozyme and Pediococcus re-inoculation after 2-3 weeks Monitor VA as indicator of spoilage bacteria PCR analysis Post Fermentation MLFML completion Malic acid < 30-50 mg/L Confirm with enzymatic assay or external lab analysis before & Aging making SO2 addition. -

The Wine List
The Wine List Here at Balzem we have taken extra time to design a wine program that celebrates the artisans, farmers and passionate winemakers who have chosen to make a little bit of wine that is unique, hand-made, true to its terroir and delicious rather than making giant amounts of wine that all tastes the same to please the masses. Champagne, Sparkling and Rosé Wines. page 1 ~~~~~~ Light & Crisp White Wines. page 2 Medium Bodied & Smooth White Wines. page 3 Full Bodied & Rich White Wines. page 4 ~~~~~~ Light & Aromatic Red Wines. page 5 Medium Bodied & Smooth Red Wines. .page 6 Full Bodied & Rich Red Wines. page 7 and 8 ~~~~~~ Seasonal Selections. page 9 California Beauties, Dessert Wines . page 10 and 11 Cocktails & Beer. page 12 Champagne & Sparkling Wines #02. Saumur Rosé N.V. Louis de Grenelle, Loire ValleY – FR 17/glass; 67/bottle #03. Prosecco 2019 Scarpetta, Friuli – IT 57/bottle #04. Pinot Meunier, Champagne, Brut N.V. Jose Michel, Champagne – FR 89/bottle Rosé Wine #06. Côtes de Provence, Quinn Rosé 2019 Provence – FR 17/glass; 57/bottle #07. Côtes de Provence, Domaine Jacourette 2016 Magnum (1,5L) Provence – FR 73/Magnum 1 Light & Crisp White Wines On this page you will find wines that are fresh, dry and bright they typically pair well with warm days, seafood or the sipper who prefers dry, crisp, bright wines. The smells and flavors are a range of citrus notes and wild flowers. Try these if you like Sauvignon Blanc or Pinot Grigio #08. Verdejo, Bodegas Menade 2019 (Sustainable) Rueda – SP 13/glass #09. -

Château Simone
Château Simone This historic estate, situated in the hills just south of Aix-en-Provence, has been in the hands of four generations of the Rougier family since 1830 and holds a virtual monopoly on the appellation of Palette. The property totals 120 hectares, with 28 under vine, of which 23 qualify for the Palette AOC. Its vineyards sit on limestone soils at elevations between 500 and 750 feet on the slopes of the Montaiguet, whose special microclimate is influenced by the encircling pine forests, the mass of the Mont Sainte-Victoire, and the Arc River. The vineyards were reconstituted after phylloxera and many vines are over a century old. In an industry that moves quicker than ever and presents ever-increasing novelty, an estate like Simone is an anchor of meaning and a lens into Provence's patrimony. Viticulture: • Farming: Ecocert Organic Certification Pending. Viticulture has always been practicing organic. • Treatments : No herbicides, chemical fertilizers, or synthetic treatments • Ploughing: Extensive ploughing and working of the soil by tractor. • Soils: Limestone Scree • Vines: Head-trained vines, many over 100-years old, that are replanted on a vine-by-vine basis to maintain a healthy vineyard • Yields: Old vines and extensive debudding lower yields and eliminate the need for a green harvest. • Harvest: Entirely manual harvest, grapes sorted in the vineyard and sorted again in the cellar • Purchasing: Always entirely estate fruit Vinification: Aging: • Fermentation: All wines are fermented spontaneously in large • Élevage: Palette wines are stored in foudres for 18 months; wooden foudres with temperature control for 2 weeks. red and white wines spend an additional year in neutral barrel.