Chromatin Accessibility Promotes Hematopoietic and Leukemia Stem Cell Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Activation of ATM Depends on Chromatin Interactions Occurring Before Induction of DNA Damage
LETTERS Activation of ATM depends on chromatin interactions occurring before induction of DNA damage Yong-Chul Kim1,6, Gabi Gerlitz1,6, Takashi Furusawa1, Frédéric Catez1,5, Andre Nussenzweig2, Kyu-Seon Oh3, Kenneth H. Kraemer3, Yosef Shiloh4 and Michael Bustin1,7 Efficient and correct responses to double-stranded breaks the KAP-1 protein9. The local and global changes in chromatin organiza- (DSB) in chromosomal DNA are crucial for maintaining genomic tion facilitate recruitment of damage-response proteins and remodelling stability and preventing chromosomal alterations that lead to factors, which further modify chromatin in the vicinity of the DSB and cancer1,2. The generation of DSB is associated with structural propagate the DNA damage response. Given the extensive changes in chro- changes in chromatin and the activation of the protein kinase matin structure associated with the DSB response, it could be expected ataxia-telangiectasia mutated (ATM), a key regulator of the that architectural chromatin-binding proteins, such as the high mobility signalling network of the cellular response to DSB3,4. The group (HMG) proteins, would be involved in this process10–12. interrelationship between DSB-induced changes in chromatin HMG is a superfamily of nuclear proteins that bind to chromatin architecture and the activation of ATM is unclear4. Here we show without any obvious specificity for a particular DNA sequence and affect that the nucleosome-binding protein HMGN1 modulates the the structure and activity of the chromatin fibre10,12,13. We have previ- interaction of ATM with chromatin both before and after DSB ously reported that HMGN1, an HMG protein that binds specifically to formation, thereby optimizing its activation. -

Functional Compensation Among HMGN Variants Modulates the Dnase I Hypersensitive Sites at Enhancers
Downloaded from genome.cshlp.org on October 9, 2021 - Published by Cold Spring Harbor Laboratory Press Research Functional compensation among HMGN variants modulates the DNase I hypersensitive sites at enhancers Tao Deng,1,12 Z. Iris Zhu,2,12 Shaofei Zhang,1 Yuri Postnikov,1 Di Huang,2 Marion Horsch,3 Takashi Furusawa,1 Johannes Beckers,3,4,5 Jan Rozman,3,5 Martin Klingenspor,6,7 Oana Amarie,3,8 Jochen Graw,3,8 Birgit Rathkolb,3,5,9 Eckhard Wolf,9 Thure Adler,3 Dirk H. Busch,10 Valérie Gailus-Durner,3 Helmut Fuchs,3 Martin Hrabeˇ de Angelis,3,4,5 Arjan van der Velde,2,13 Lino Tessarollo,11 Ivan Ovcherenko,2 David Landsman,2 and Michael Bustin1 1Protein Section, Laboratory of Metabolism, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892, USA; 2Computational Biology Branch, National Center for Biotechnology Information, National Library of Medicine, Bethesda, Maryland 20892, USA; 3German Mouse Clinic, Institute of Experimental Genetics, Helmholtz Zentrum München, German Research Center for Environmental Health, 85764 Neuherberg, Germany; 4Experimental Genetics, Center of Life and Food Sciences Weihenstephan, Technische Universität München, 85354 Freising-Weihenstephan, Germany; 5German Center for Diabetes Research (DZD), 85764 Neuherberg, Germany; 6Molecular Nutritional Medicine, Technische Universität München, 85350 Freising, Germany; 7Center for Nutrition and Food Sciences, Technische Universität München, 85350 Freising, Germany; 8Institute of Developmental Genetics (IDG), 85764 -

Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model
Downloaded from http://www.jimmunol.org/ by guest on September 25, 2021 T + is online at: average * The Journal of Immunology , 34 of which you can access for free at: 2016; 197:1477-1488; Prepublished online 1 July from submission to initial decision 4 weeks from acceptance to publication 2016; doi: 10.4049/jimmunol.1600589 http://www.jimmunol.org/content/197/4/1477 Molecular Profile of Tumor-Specific CD8 Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. Waugh, Sonia M. Leach, Brandon L. Moore, Tullia C. Bruno, Jonathan D. Buhrman and Jill E. Slansky J Immunol cites 95 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2016/07/01/jimmunol.160058 9.DCSupplemental This article http://www.jimmunol.org/content/197/4/1477.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 25, 2021. The Journal of Immunology Molecular Profile of Tumor-Specific CD8+ T Cell Hypofunction in a Transplantable Murine Cancer Model Katherine A. -

Homeobox Gene Methylation in Lung Cancer Studied by Genome-Wide Analysis with a Microarray-Based Methylated Cpg Island Recovery Assay
Homeobox gene methylation in lung cancer studied by genome-wide analysis with a microarray-based methylated CpG island recovery assay Tibor Rauch*, Zunde Wang*, Xinmin Zhang†, Xueyan Zhong*, Xiwei Wu‡, Sean K. Lau§, Kemp H. Kernstine¶, Arthur D. Riggs*ʈ, and Gerd P. Pfeifer*ʈ *Division of Biology, ‡Division of Information Sciences, §Division of Pathology, and ¶Division of Surgery, Beckman Research Institute of the City of Hope, Duarte, CA 91010; and †NimbleGen Systems, Inc., Madison, WI 53711 Contributed by Arthur D. Riggs, February 5, 2007 (sent for review December 11, 2006) De novo methylation of CpG islands is a common phenomenon in and in this way they are key determinants of cell identity and human cancer, but the mechanisms of cancer-associated DNA potential targets during tumorigenesis. methylation are not known. We have used tiling arrays in combi- We recently developed a DNA methylation detection technique, nation with the methylated CpG island recovery assay to investi- the methylated CpG island recovery assay (MIRA) (12). This gate methylation of CpG islands genome-wide and at high reso- technique is based on the high affinity of a complex of the MBD2b lution. We find that all four HOX gene clusters on chromosomes 2, and MBD3L1 proteins for CpG-methylated DNA (13) and is 7, 12, and 17 are preferential targets for DNA methylation in cancer compatible with microarray-based methodology (14). We previ- cell lines and in early-stage lung cancer. CpG islands associated ously found that several homeobox genes were methylated in a lung with many other homeobox genes, such as SIX, LHX, PAX, DLX, and cancer cell line (14). -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Continuous MLL-ENL Expression Is Necessary to Establish a ''Hox Code'' and Maintain Immortalization of Hematopoietic Progenitor Cells
Research Article Continuous MLL-ENL Expression Is Necessary to Establish a ‘‘Hox Code’’ and Maintain Immortalization of Hematopoietic Progenitor Cells Sarah J. Horton,1 David G. Grier,3 Glenda J. McGonigle,3 Alexander Thompson,3 Michelle Morrow,1 Inusha De Silva,1 Dale A. Moulding,1 Dimitris Kioussis,2 Terence R.J. Lappin,3 Hugh J.M. Brady,1 and Owen Williams1 1Molecular Haematology and Cancer Biology Unit, Institute of Child Health, University College London; 2Division of Molecular Immunology, National Institute for Medical Research, London, United Kingdom; and 3Department of Child Health, Queen’s University, Belfast, United Kingdom Abstract essential for definitive hematopoiesis and that it is required to The t[(11;19)(p22;q23)] translocation, which gives rise to the maintain, but not to initiate, the expression of multiple Hox genes MLL-ENL fusion protein, is commonly found in infant acute during embryogenesis (5–9). Some Hox genes are oncogenic when leukemias of both the myeloid and lymphoid lineage. To overexpressed in hematopoietic progenitor cells (10, 11). Taken investigate the molecular mechanism of immortalization by together, this suggests that aberrant regulation of Hox genes by MLL-ENL we established a Tet-regulatable system of MLL- MLL fusion proteins is the basis for leukemias involving MLL ENL expression in primary hematopoietic progenitor cells. translocations (12). Several recent publications do indeed suggest Immortalized myeloid cell lines were generated, which that Hox genes may play an important role in leukemia induced by are dependent on continued MLL-ENL expression for their MLL fusion proteins (13–16). survival and proliferation. These cells either terminally MLL translocations result in the generation of an in-frame differentiate or die when MLL-ENL expression is turned chimeric fusion in which MLL is joined to one of over 40 different off with doxycycline. -

Rabbit Anti-HOXA6/FITC Conjugated Antibody
SunLong Biotech Co.,LTD Tel: 0086-571- 56623320 Fax:0086-571- 56623318 E-mail:[email protected] www.sunlongbiotech.com Rabbit Anti-HOXA6/FITC Conjugated antibody SL11294R-FITC Product Name: Anti-HOXA6/FITC Chinese Name: FITC标记的同源盒基因HOXA6蛋白抗体 HOX1B; Homeo box 1B; Homeo box A6; Homeobox 1B; Homeobox A6; Homeobox protein Hox A6; Homeobox protein Hox-1B; Homeobox protein Hox-A6 antibody Alias: Homeobox protein HoxA6; HOX 1; Hox 1B; HOX1; HOX1.2; Hox1B; HOXA6; HX A6; HXA6; HXA6_HUMAN. Organism Species: Rabbit Clonality: Polyclonal React Species: Human,Mouse,Rat,Dog,Pig,Horse,Sheep, ICC=1:50-200IF=1:50-200 Applications: not yet tested in other applications. optimal dilutions/concentrations should be determined by the end user. Molecular weight: 23kDa Form: Lyophilized or Liquid Concentration: 1mg/ml immunogen: KLH conjugated synthetic peptide derived from human HOXA6/HOX1B (171-233aa) Lsotype: IgG Purification: affinitywww.sunlongbiotech.com purified by Protein A Storage Buffer: 0.01M TBS(pH7.4) with 1% BSA, 0.03% Proclin300 and 50% Glycerol. Store at -20 °C for one year. Avoid repeated freeze/thaw cycles. The lyophilized antibody is stable at room temperature for at least one month and for greater than a year Storage: when kept at -20°C. When reconstituted in sterile pH 7.4 0.01M PBS or diluent of antibody the antibody is stable for at least two weeks at 2-4 °C. background: The Hox homeobox genes encode proteins that are transcriptional regulators with an established role in embryonic development. HoxA6 (homeobox A6), also known as Product Detail: HOX1B, is a 233 amino acid protein that localizes to the nucleus. -

HMGB1 in Health and Disease R
Donald and Barbara Zucker School of Medicine Journal Articles Academic Works 2014 HMGB1 in health and disease R. Kang R. C. Chen Q. H. Zhang W. Hou S. Wu See next page for additional authors Follow this and additional works at: https://academicworks.medicine.hofstra.edu/articles Part of the Emergency Medicine Commons Recommended Citation Kang R, Chen R, Zhang Q, Hou W, Wu S, Fan X, Yan Z, Sun X, Wang H, Tang D, . HMGB1 in health and disease. 2014 Jan 01; 40():Article 533 [ p.]. Available from: https://academicworks.medicine.hofstra.edu/articles/533. Free full text article. This Article is brought to you for free and open access by Donald and Barbara Zucker School of Medicine Academic Works. It has been accepted for inclusion in Journal Articles by an authorized administrator of Donald and Barbara Zucker School of Medicine Academic Works. Authors R. Kang, R. C. Chen, Q. H. Zhang, W. Hou, S. Wu, X. G. Fan, Z. W. Yan, X. F. Sun, H. C. Wang, D. L. Tang, and +8 additional authors This article is available at Donald and Barbara Zucker School of Medicine Academic Works: https://academicworks.medicine.hofstra.edu/articles/533 NIH Public Access Author Manuscript Mol Aspects Med. Author manuscript; available in PMC 2015 December 01. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Mol Aspects Med. 2014 December ; 0: 1–116. doi:10.1016/j.mam.2014.05.001. HMGB1 in Health and Disease Rui Kang1,*, Ruochan Chen1, Qiuhong Zhang1, Wen Hou1, Sha Wu1, Lizhi Cao2, Jin Huang3, Yan Yu2, Xue-gong Fan4, Zhengwen Yan1,5, Xiaofang Sun6, Haichao Wang7, Qingde Wang1, Allan Tsung1, Timothy R. -

TRPV4: a Physio and Pathophysiologically Significant Ion Channel
International Journal of Molecular Sciences Review TRPV4: A Physio and Pathophysiologically Significant Ion Channel Tamara Rosenbaum 1,* , Miguel Benítez-Angeles 1, Raúl Sánchez-Hernández 1, Sara Luz Morales-Lázaro 1, Marcia Hiriart 1 , Luis Eduardo Morales-Buenrostro 2 and Francisco Torres-Quiroz 3 1 Departamento de Neurociencia Cognitiva, División Neurociencias, Instituto de Fisiología Celular, Universidad Nacional Autónoma de México, Mexico City 04510, Mexico; [email protected] (M.B.-A.); [email protected] (R.S.-H.); [email protected] (S.L.M.-L.); [email protected] (M.H.) 2 Departamento de Nefrología y Metabolismo Mineral, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City 14080, Mexico; [email protected] 3 Departamento de Bioquímica y Biología Estructural, División Investigación Básica, Instituto de Fisiología Celular, Universidad Nacional Autónoma de México, Mexico City 04510, Mexico; [email protected] * Correspondence: [email protected]; Tel.: +52-555-622-56-24; Fax: +52-555-622-56-07 Received: 3 May 2020; Accepted: 24 May 2020; Published: 28 May 2020 Abstract: Transient Receptor Potential (TRP) channels are a family of ion channels whose members are distributed among all kinds of animals, from invertebrates to vertebrates. The importance of these molecules is exemplified by the variety of physiological roles they play. Perhaps, the most extensively studied member of this family is the TRPV1 ion channel; nonetheless, the activity of TRPV4 has been associated to several physio and pathophysiological processes, and its dysfunction can lead to severe consequences. Several lines of evidence derived from animal models and even clinical trials in humans highlight TRPV4 as a therapeutic target and as a protein that will receive even more attention in the near future, as will be reviewed here. -

Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association Between BMI and Adult-Onset Non- Atopic
Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association between BMI and Adult-Onset Non- Atopic Asthma Ayoung Jeong 1,2, Medea Imboden 1,2, Akram Ghantous 3, Alexei Novoloaca 3, Anne-Elie Carsin 4,5,6, Manolis Kogevinas 4,5,6, Christian Schindler 1,2, Gianfranco Lovison 7, Zdenko Herceg 3, Cyrille Cuenin 3, Roel Vermeulen 8, Deborah Jarvis 9, André F. S. Amaral 9, Florian Kronenberg 10, Paolo Vineis 11,12 and Nicole Probst-Hensch 1,2,* 1 Swiss Tropical and Public Health Institute, 4051 Basel, Switzerland; [email protected] (A.J.); [email protected] (M.I.); [email protected] (C.S.) 2 Department of Public Health, University of Basel, 4001 Basel, Switzerland 3 International Agency for Research on Cancer, 69372 Lyon, France; [email protected] (A.G.); [email protected] (A.N.); [email protected] (Z.H.); [email protected] (C.C.) 4 ISGlobal, Barcelona Institute for Global Health, 08003 Barcelona, Spain; [email protected] (A.-E.C.); [email protected] (M.K.) 5 Universitat Pompeu Fabra (UPF), 08002 Barcelona, Spain 6 CIBER Epidemiología y Salud Pública (CIBERESP), 08005 Barcelona, Spain 7 Department of Economics, Business and Statistics, University of Palermo, 90128 Palermo, Italy; [email protected] 8 Environmental Epidemiology Division, Utrecht University, Institute for Risk Assessment Sciences, 3584CM Utrecht, Netherlands; [email protected] 9 Population Health and Occupational Disease, National Heart and Lung Institute, Imperial College, SW3 6LR London, UK; [email protected] (D.J.); [email protected] (A.F.S.A.) 10 Division of Genetic Epidemiology, Medical University of Innsbruck, 6020 Innsbruck, Austria; [email protected] 11 MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, W2 1PG London, UK; [email protected] 12 Italian Institute for Genomic Medicine (IIGM), 10126 Turin, Italy * Correspondence: [email protected]; Tel.: +41-61-284-8378 Int. -
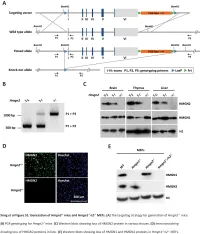
Supplemental Data.Pdf
Table S1. Summary of sequencing results A. DNase‐seq data % Align % Mismatch % >=Q30 bases Sample ID # Reads % unique reads (PF) Rate (PF) (PF) WT_1 84,301,522 86.76 0.52 93.34 96.30 WT_2 98,744,222 84.97 0.51 93.75 89.94 Hmgn1‐/‐_1 79,620,656 83.94 0.86 90.58 88.65 Hmgn1‐/‐_2 62,673,782 84.13 0.87 91.30 89.18 Hmgn2‐/‐_1 87,734,440 83.49 0.71 91.81 90.00 Hmgn2‐/‐_2 82,498,808 83.25 0.69 92.73 90.66 Hmgn1‐/‐n2‐/‐_1 71,739,638 68.51 2.31 81.11 89.22 Hmgn1‐/‐n2‐/‐_2 74,113,682 68.19 2.37 81.16 86.57 B. ChIP‐seq data Histone % Align % Mismatch % >=Q30 % unique Genotypes # Reads marks (PF) Rate (PF) bases (PF) reads H3K4me1 100670054 92.99 0.28 91.21 87.29 H3K4me3 67064272 91.97 0.35 89.11 27.15 WT H3K27ac 90,340,242 93.57 0.28 95.02 89.80 input 111,292,572 78.24 0.55 96.07 86.99 H3K4me1 84598176 92.34 0.33 91.2 81.69 H3K4me3 90032064 92.19 0.44 88.76 15.81 Hmgn1‐/‐n2‐/‐ H3K27ac 86,260,526 93.40 0.29 94.94 87.49 input 78,142,334 78.47 0.56 95.82 81.11 C. MNase‐seq data % Mismatch % >=Q30 bases % unique Sample ID # Reads % Align (PF) Rate (PF) (PF) reads WT_1_Extensive 45,232,694 55.23 1.49 90.22 81.73 WT_1_Limited 105,460,950 58.03 1.39 90.81 79.62 WT_2_Extensive 40,785,338 67.34 1.06 89.76 89.60 WT_2_Limited 105,738,078 68.34 1.05 90.29 85.96 Hmgn1‐/‐n2‐/‐_1_Extensive 117,927,050 55.74 1.49 89.50 78.01 Hmgn1‐/‐n2‐/‐_1_Limited 61,846,742 63.76 1.22 90.57 84.55 Hmgn1‐/‐n2‐/‐_2_Extensive 137,673,830 60.04 1.30 89.28 78.99 Hmgn1‐/‐n2‐/‐_2_Limited 45,696,614 62.70 1.21 90.71 85.52 D. -

Mapping of SUMO Sites and Analysis of Sumoylation Changes Induced by External Stimuli
Mapping of SUMO sites and analysis of SUMOylation changes induced by external stimuli Francis Impensa,b,c, Lilliana Radoshevicha,b,c, Pascale Cossarta,b,c,1, and David Ribeta,b,c,1 aUnité des Interactions Bactéries-Cellules, Institut Pasteur, F-75015 Paris, France; bInstitut National de la Santé et de la Recherche Médicale, Unité 604, F-75015 Paris, France; and cInstitut National de la Recherche Agronomique, Unité sous-contrat 2020, F-75015 Paris, France Contributed by Pascale Cossart, July 22, 2014 (sent for review May 28, 2014) SUMOylation is an essential ubiquitin-like modification involved in Mapping the exact lysine residue to which SUMO is attached in important biological processes in eukaryotic cells. Identification of modified proteins is a critical step to get further insight into the small ubiquitin-related modifier (SUMO)-conjugatedresiduesinpro- function of SUMOylation. Indeed, the identification of SUMO teins is critical for understanding the role of SUMOylation but remains sites allows the generation of non-SUMOylatable mutants and the experimentally challenging. We have set up a powerful and high- study of associated phenotypes. Identification of SUMO sites by throughput method combining quantitative proteomics and peptide MS is not straightforward (8). Unlike ubiquitin, which leaves immunocapture to map SUMOylation sites and have analyzed changes a small diglycine (GG) signature tag on the modified lysine resi- in SUMOylation in response to stimuli. With this technique we iden- due after trypsin digestion, SUMO leaves