Traumatic Injuries to the Spinal Cord and Peripheral Nervous System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Positive Cases in Suspected Cauda Equina Syndrome
Edinburgh Research Explorer The clinical features and outcome of scan-negative and scan- positive cases in suspected cauda equina syndrome Citation for published version: Hoeritzauer, I, Pronin, S, Carson, A, Statham, P, Demetriades, AK & Stone, J 2018, 'The clinical features and outcome of scan-negative and scan-positive cases in suspected cauda equina syndrome: a retrospective study of 276 patients', Journal of Neurology, vol. 265, no. 12. https://doi.org/10.1007/s00415- 018-9078-2 Digital Object Identifier (DOI): 10.1007/s00415-018-9078-2 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Journal of Neurology Publisher Rights Statement: This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 04. Oct. 2021 Journal of Neurology (2018) 265:2916–2926 https://doi.org/10.1007/s00415-018-9078-2 ORIGINAL COMMUNICATION The clinical features and outcome of scan-negative and scan-positive cases in suspected cauda equina syndrome: a retrospective study of 276 patients Ingrid Hoeritzauer1,2,5 · Savva Pronin1,5 · Alan Carson1,2,3 · Patrick Statham2,4,5 · Andreas K. -

Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection
Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection This guide is for middle and high school students participating in AIMS Anatomy of the Human Brain and Sheep Brain Dissections. Programs will be presented by an AIMS Anatomy Specialist. In this activity students will become more familiar with the anatomical structures of the human brain by observing, studying, and examining human specimens. The primary focus is on the anatomy, function, and pathology. Those students participating in Sheep Brain Dissections will have the opportunity to dissect and compare anatomical structures. At the end of this document, you will find anatomical diagrams, vocabulary review, and pre/post tests for your students. The following topics will be covered: 1. The neurons and supporting cells of the nervous system 2. Organization of the nervous system (the central and peripheral nervous systems) 4. Protective coverings of the brain 5. Brain Anatomy, including cerebral hemispheres, cerebellum and brain stem 6. Spinal Cord Anatomy 7. Cranial and spinal nerves Objectives: The student will be able to: 1. Define the selected terms associated with the human brain and spinal cord; 2. Identify the protective structures of the brain; 3. Identify the four lobes of the brain; 4. Explain the correlation between brain surface area, structure and brain function. 5. Discuss common neurological disorders and treatments. 6. Describe the effects of drug and alcohol on the brain. 7. Correctly label a diagram of the human brain National Science Education -

A Rare Case of Penetrating Trauma of Frontal Sinus with Anterior Table Fracture Himanshu Raval1*, Mona Bhatt2 and Nihar Gaur3
ISSN: 2643-4474 Raval et al. Neurosurg Cases Rev 2020, 3:046 DOI: 10.23937/2643-4474/1710046 Volume 3 | Issue 2 Neurosurgery - Cases and Reviews Open Access CASE REPORT Case Report: A Rare Case of Penetrating Trauma of Frontal Sinus with Anterior Table Fracture Himanshu Raval1*, Mona Bhatt2 and Nihar Gaur3 1 Department of Neurosurgery, NHL Municipal Medical College, SVP Hospital Campus, Gujarat, India Check for updates 2Medical Officer, CHC Dolasa, Gujarat, India 3GAIMS-GK General Hospital, Gujarat, India *Corresponding author: Dr. Himanshu Raval, Resident, Department of Neurosurgery, NHL Municipal Medical College, SVP Hospital Campus, Elisbridge, Ahmedabad, Gujarat, 380006, India, Tel: 942-955-3329 Abstract Introduction Background: Head injury is common component of any Road traffic accident (RTA) is the most common road traffic accident injury. Injury involving only frontal sinus cause of cranio-facial injury and involvement of frontal is uncommon and unique as its management algorithm is bone fractures are rare and constitute 5-9% of only fa- changing over time with development of radiological modal- ities as well as endoscopic intervention. Frontal sinus inju- cial trauma. The degree of association has been report- ries may range from isolated anterior table fractures causing ed to be 95% with fractures of the anterior table or wall a simple aesthetic deformity to complex fractures involving of the frontal sinuses, 60% with the orbital rims, and the frontal recess, orbits, skull base, and intracranial con- 60% with complex injuries of the naso-orbital-ethmoid tents. Only anterior table injury of frontal sinus is rare in pen- region, 33% with other orbital wall fractures and 27% etrating head injury without underlying brain injury with his- tory of unconsciousness and questionable convulsion which with Le Fort level fractures. -

Cardiovascular Collapse Following Succinylcholine in a Paraplegic Patient
ParajJleg£a (I973), II, 199-204 CARDIOVASCULAR COLLAPSE FOLLOWING SUCCINYLCHOLINE IN A PARAPLEGIC PATIENT By J. C. SNOW,! M.D., B. J. KRIPKE, M.D. , G. P. SESSIONS, M.D. and A. J. FINCK, M.D. University Hospital, Boston, DeKalb General Hospital, Decatur, Georgia, and Boston University School of Medicine, Boston, Massachusetts 02118 INTRODUCTION SEVERAL reports have been presented that have discussed cardiovascular collapse following the intravenous infusion of succinylcholine in patients with burns, massive trauma,tetanus, spinal cord injury, brain injury,upper or lower motor neuron disease,and uraemia with increased serum potassium. The purpose of this article is to report a spinal cord injured patient who developed cardiac arrest following administration of succinylcholine, possibly due to succinylcholine-induced hyperkalaemia. The anaesthesia management during the course of subsequent surgical procedure proved to be uneventful. CASE REPORT On 7 December 1971, a 20-year-old white man was admitted to the hospital after he fell 50 feet from a scaffold to the ground. He was reported to have been in good health until this accident. His legs and outstretched hands absorbed the major impact. No loss of consciousness was reported at any time. Neurologic examination revealed absent function of muscle groups in the distribution distal to L4, including the sacral segments. There were contractions of both quadriceps muscles in the adductors of the legs. The legs were held in flexion with no evidence of function in his hip abductors, extensors, knee flexors or anything below his knee. He had intact sensation over the entire thigh and medial calf. He had no apparent abdominal or cremasteric reflexes, no knee or ankle jerks, no Babinski responses, and no sacral sparing. -

Power Wheelchair Alternative Drive Controls in Spinal Cord Injury
Power Wheelchair Alternative Drive Controls in Spinal Cord Injury Kristen Cezat, PT, DPT, NCS, ATP/SMS When a person with a spinal cord injury is unable to use a standard joystick on a Fact Sheet power wheelchair, an alternative drive control and location is required. Depending on the person’s level of injury and musculature that remains innervated, the more common locations of alternative drive control input devices include the head, neck, face, eyes, and tongue. A proportional drive control allows the wheelchair’s speed/direction to mirror the force/direction applied to the input device by the user, most often through a joystick. Proportional drive control options include1: • Standard Joystick: able to move in any direction at any speed. It is often the most intuitive device for adults with injuries at C5 and below. Joysticks are commonly located on either left or right arm rest but can be altered for Produced by location in midline if spasticity or contracture limits neutral shoulder/elbow alignment. • Alternative Joystick (often set at the user’s chin): joysticks vary in size and require less force and deflection to activate the joystick.1 air Drive ControlA non -Optionsproportional drive for control Clients uses commands with to turn on/offSpinal various functionsCord such as direction (forward, back, left, or right) of the wheelchair. Speed is predetermined and not variable to the strength of the command provided. Non- Injury proportional drive controls include1,2: a Special Interest • Sip-and-Puff Group of • Head Array • Head Array/Sip-and-Puff Combo Non-proportional drive controls can be either momentary or latched. -

The Effect of the Moufarrege Total Posterior Pedicle Reduction Mammaplasty on the Erogenous Sensation of the Nipple
Surgical Science, 2019, 10, 127-140 http://www.scirp.org/journal/ss ISSN Online: 2157-9415 ISSN Print: 2157-9407 The Effect of the Moufarrege Total Posterior Pedicle Reduction Mammaplasty on the Erogenous Sensation of the Nipple Richard Moufarrege1,2*, Mohammed El Mehdi El Yamani1, Laura Barriault1, Ahmed Amine Alaoui1 1Faculty of Medicine, Université de Montréal, Montreal, Canada 2Department of Plastic Surgery, Université de Montréal, Montreal, Canada How to cite this paper: Moufarrege, R., El Abstract Yamani, M.E.M., Barriault, L. and Alaoui, A.A. (2019) The Effect of the Moufarrege Traditional reduction mammoplasties have the simple concern to guarantee Total Posterior Pedicle Reduction Mam- the survival of the nipple areola complex after surgery. Little has been done to maplasty on the Erogenous Sensation of the take care of essential functions in the nipple, especially the erogenous sensa- Nipple. Surgical Science, 10, 127-140. https://doi.org/10.4236/ss.2019.104016 tion. We have conducted a retrospective study on a cohort of 573 female pa- tients operated using the Total Posterior Pedicle of Moufarrege between 1985 Received: February 25, 2019 and 1995 to evaluate its effect on the erogenous sensation of the nipple. This Accepted: April 23, 2019 study demonstrated the preservation of the erogenous sensation of the nipple Published: April 26, 2019 in a high proportion of these patients. The physiology of this preservation is Copyright © 2019 by author(s) and explained in regard of the technique details in Moufarrege mammoplasty Scientific Research Publishing Inc. compared to other techniques. The Moufarrege Total Posterior Pedicle would This work is licensed under the Creative therefore be a highly reliable reduction technique to ensure the preservation Commons Attribution International License (CC BY 4.0). -
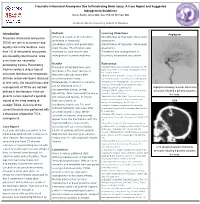
Traumatic Intracranial Aneurysms Due to Penetrating Brain Injury. a Case Report and Suggested Management Guidelines Breck Aaron Jones MD; Alex Patrick Michael MD
Traumatic Intracranial Aneurysms Due to Penetrating Brain Injury. A Case Report and Suggested Management Guidelines Breck Aaron Jones MD; Alex Patrick Michael MD Southern Illinois University School of Medicine Methods Learning Objectives Introduction Angiogram Traumatic intracranial aneurysms A Pubmed search of the literature Identification of traumatic intracranial pertaining to traumatic aneurysms. (TICA) are rare in occurrence and pseudoaneurysms and penetrating Classification of traumatic intracranial equally rare in the literature. Less brain trauma. The literature was aneurysms. than 1% of intracranial aneurysms reviewed for case reports and Treatment and management of are caused by blunt trauma, while management recommendations. traumatic intracranial aneurysms. even fewer are caused by penetrating trauma. Penetrating Results References Traumatic intracranial aneurysm 1.Aarabi B. Management of traumatic aneurysms caused by trauma creates a unique type of high-velocity missile head wounds. Neurosurg Clin N Am. formation is the most commonly aneurysm that does not incorporate Oct 1995;6(4):775-797. described vascular injury after 2.Rao GP, Rao NS, Reddy PK. Technique of removal of an all three vessel wall layers. Because penetrating brain injury. impacted sharp object in a penetrating head injury using the of their rarity, the natural history and Histologically, traumatic aneurysms lever principle. Br J Neurosurg. Dec 1998;12(6):569-571. 3.Vascular complications of penetrating brain injury. J can be described as true management of TICAs are not well Trauma. Aug 2001;51(2 Suppl):S26-28. Angiogram showing traumatic intracranial (incorporating intima, media, defined in the literature. Here we 4.Crompton MR. The pathogenesis of cerebral aneurysms. aneurysm following a gunshot wound to adventitia), false (incorporating one or Brain. -

Crush Injuries Pathophysiology and Current Treatment Michael Sahjian, RN, BSN, CFRN, CCRN, NREMT-P; Michael Frakes, APRN, CCNS, CCRN, CFRN, NREMT-P
LWW/AENJ LWWJ331-02 April 23, 2007 13:50 Char Count= 0 Advanced Emergency Nursing Journal Vol. 29, No. 2, pp. 145–150 Copyright c 2007 Wolters Kluwer Health | Lippincott Williams & Wilkins Crush Injuries Pathophysiology and Current Treatment Michael Sahjian, RN, BSN, CFRN, CCRN, NREMT-P; Michael Frakes, APRN, CCNS, CCRN, CFRN, NREMT-P Abstract Crush syndrome, or traumatic rhabdomyolysis, is an uncommon traumatic injury that can lead to mismanagement or delayed treatment. Although rhabdomyolysis can result from many causes, this article reviews the risk factors, symptoms, and best practice treatments to optimize patient outcomes, as they relate to crush injuries. Key words: crush syndrome, traumatic rhabdomyolysis RUSH SYNDROME, also known as ology, pathophysiology, diagnosis, and early traumatic rhabdomyolysis, was first re- management of crush syndrome. Cported in 1910 by German authors who described symptoms including muscle EPIDEMIOLOGY pain, weakness, and brown-colored urine in soldiers rescued after being buried in struc- Crush injuries may result in permanent dis- tural debris (Gonzalez, 2005). Crush syn- ability or death; therefore, early recognition drome was not well defined until the 1940s and aggressive treatment are necessary to when nephrologists Bywaters and Beal pro- improve outcomes. There are many known vided descriptions of victims trapped by mechanisms inducing rhabdomyolysis includ- their extremities during the London Blitz ing crush injuries, electrocution, burns, com- who presented with shock, swollen extrem- partment syndrome, and any other pathology ities, tea-colored urine, and subsequent re- that results in muscle damage. Victims of nat- nal failure (Better & Stein, 1990; Fernan- ural disasters, including earthquakes, are re- dez, Hung, Bruno, Galea, & Chiang, 2005; ported as having up to a 20% incidence of Gonzalez, 2005; Malinoski, Slater, & Mullins, crush injuries, as do 40% of those surviving to 2004). -

Wallerian Degeneration and Inflammation in Rat Peripheral Nerve Detected by in Vivo MR Imaging
741 Wallerian Degeneration and Inflammation in Rat Peripheral Nerve Detected by in Vivo MR Imaging DavidS. Titelbaum 1 To investigate the role of MR imaging in wallerian degeneration, a series of animal Joel L. Frazier 2 models of increasingly complex peripheral nerve injury were studied by in vivo MR. Robert I. Grossman 1 Proximal tibial nerves in brown Norway rats were either crushed, transected (neurotomy), Peter M. Joseph 1 or transected and grafted with Lewis rat (allograft) or brown Norway (isograft) donor Leonard T. Yu 2 nerves. The nerves distal to the site of injury were imaged at intervals of 0-54 days after surgery. Subsequent histologic analysis was obtained and correlated with MR Eleanor A. Kassab 1 3 findings. Crush injury, neurotomy, and nerve grafting all resulted in high signal intensity William F. Hickey along the course of the nerve observed on long TR/TE sequences, corresponding to 2 Don LaRossa edema and myelin breakdown from wallerian degeneration. The abnormal signal inten 4 Mark J. Brown sity resolved by 30 days after crush injury and by 45-54 days after neurotomy, when the active changes of wallerian degeneration had subsided. These changes were not seen in sham-operated rats. Our findings suggest that MR is capable of identifying traumatic neuropathy in a peripheral nerve undergoing active wallerian degeneration. The severity of injury may be reflected by the corresponding duration of signal abnormality. With the present methods, MR did not distinguish inflammatory from simple posttraumatic neuropathy. Wallerian degeneration is the axonal degeneration and loss of myelin that occurs when an axon is separated from its cell body. -

Blunt and Blast Head Trauma: Different Entities
International Tinnitus Journal, Vol. 15, No. 2, 115–118 (2009) Blunt and Blast Head Trauma: Different Entities Michael E. Hoffer,1 Chadwick Donaldson,1 Kim R. Gottshall1, Carey Balaban,2 and Ben J. Balough1 1 Spatial Orientation Center, Department of Otolaryngology, Naval Medical Center San Diego, San Diego, California, and 2 Department of Otolaryngology, University of Pittsburgh, Pittsburgh, Pennsylvania, USA Abstract: Mild traumatic brain injury (mTBI) caused by blast-related and blunt head trauma is frequently encountered in clinical practice. Understanding the nuances between these two distinct types of injury leads to a more focused approach by clinicians to develop better treat- ment strategies for patients. In this study, we evaluated two separate cohorts of mTBI patients to ascertain whether any difference exists in vestibular-ocular reflex (VOR) testing (n ϭ 55 en- rolled patients: 34 blunt, 21 blast) and vestibular-spinal reflex (VSR) testing (n ϭ 72 enrolled patients: 33 blunt, 39 blast). The VOR group displayed a preponderance of patients with blunt mTBI, demonstrating normal to high-frequency phase lag on rotational chair testing, whereas patients experiencing mTBI from blast-related causes revealed a trend toward low-frequency phase lag on evaluation. The VSR cohort showed that patients with posttraumatic migraine- associated dizziness tended to test higher on posturography. However, an indepth look at the total patient population in this second cohort reveals that a higher percentage of blast-exposed patients exhibited a significantly increased latency on motor control testing as compared to pa- tients with blunt head injury ( p Ͻ .02). These experiments identify a distinct difference be- tween blunt-injury and blast-injury mTBI patients and provide evidence that treatment strategies should be individualized on the basis of each mechanism of injury. -

Suggested Osteopathic Treatment.Pdf
Suggested Osteopathic Treatment of Respiratory Diseases Processes Region Biomechanical Model Neurological Model Cardio/Resp Model Metabolic Model Behavioral Model Sample Techniques Head/OA Improve motion CN X - Improve Parasympathetic innervations affect Improve CSF flow (part Reduces anxiety associated with Sub-occipital release; OA decompression; parasympathetic balance heart rate; Improve PRM of PRM) contraction of disease Sinus Drainage (if sings of URI) C-Spine C3-5 Diaphragm C3-5 Diaphragm Assist lymph movement Reduces anxiety associated with Soft Tissue/Myofascial of C-spine, BLT, contraction of disease MET, Counterstrain Thoracic Improve rib cage Stellate Ganglion Lymph drainage (bolster immune Improve oxygenation Normalizes sympathetic drive thus Thoracic Outlet Release, 1st rib release, Outlet motion response) balancing somatopsychological pathways Sternum Improve rib cage Intercostal nerves Improve lymph flow (bolster immune Improve oxygenation Normalizes sympathetic drive thus Sternal/ C-T myofascial release motion response) (reduces work of balancing somatopsychological breathing) pathways Upper Scapula – improve rib Brachial plexus Improve lymph flow Normalizes sympathetic drive thus Scapular balancing, Spencer’s technique, Extremity cage function balancing somatopsychological MET, Counterstrain, Upper Extremity pathways Wobble technique Thoracic Improve rib cage Celiac, Inferior and Improve lymph flow Improve oxygenation Normalizes sympathetic drive thus Soft Tissue/Myofascial of T-spine or Spine motion superior mesenteric -

Case Report Forearm Compartment Syndrome Following Thrombolytic Therapy for Massive Pulmonary Embolism: a Case Report and Review of Literature
Hindawi Publishing Corporation Case Reports in Orthopedics Volume 2011, Article ID 678525, 4 pages doi:10.1155/2011/678525 Case Report Forearm Compartment Syndrome following Thrombolytic Therapy for Massive Pulmonary Embolism: A Case Report and Review of Literature Ravi Badge and Mukesh Hemmady Department of Trauma and Orthopaedics Surgery, Wrightington, Wigan and Leigh NHS Trust, Wigan WN1 2NN, UK Correspondence should be addressed to Ravi Badge, [email protected] Received 2 November 2011; Accepted 6 December 2011 Academic Editor: M. K. Lyons Copyright © 2011 R. Badge and M. Hemmady. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Use of thrombolytic therapy in pulmonary embolism is restricted in cases of massive embolism. It achieves faster lysis of the thrombus than the conventional heparin therapy thus reducing the morbidity and mortality associated with PE. The compartment syndrome is a well-documented, potentially lethal complication of thrombolytic therapy and known to occur in the limbs involved for vascular lines or venepunctures. The compartment syndrome in a conscious and well-oriented patient is mainly diagnosed on clinical ground with its classical signs and symptoms like disproportionate pain, tense swollen limb and pain on passive stretch. However these findings may not be appropriately assessed in an unconscious patient and therefore the clinicians should have high index of suspicion in a patient with an acutely swollen tense limb. In such scenarios a prompt orthopaedic opinion should be considered. In this report, we present a case of acute compartment syndrome of the right forearm in a 78 years old male patient following repeated attempts to secure an arterial line for initiating the thrombolytic therapy for the management of massive pulmonary embolism.