First-Line Treatment of Oral Candidiasis in Adults
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antibiofilm Efficacy of Tea Tree Oil and of Its Main Component Terpinen-4-Ol Against Candida Albicans
ORIGINAL RESEARCH Periodontics Antibiofilm efficacy of tea tree oil and of its main component terpinen-4-ol against Candida albicans Renata Serignoli Abstract: Candida infection is an important cause of morbidity FRANCISCONI(a) and mortality in immunocompromised patients. The increase in its Patricia Milagros Maquera incidence has been associated with resistance to antimicrobial therapy HUACHO(a) and biofilm formation. The aim of this study was to evaluate the Caroline Coradi TONON(a) efficacy of tea tree oil (TTO) and its main component – terpinen-4-ol – Ester Alves Ferreira BORDINI(a) against resistant Candida albicans strains (genotypes A and B) identified by molecular typing and against C. albicans ATCC 90028 and SC 5314 Marília Ferreira CORREIA(a) reference strains in planktonic and biofilm cultures. The minimum Janaína de Cássia Orlandi inhibitory concentration, minimum fungicidal concentration, and SARDI(b) rate of biofilm development were used to evaluate antifungal activity. Denise Madalena Palomari Results were obtained from analysis of the biofilm using the cell (a) SPOLIDORIO proliferation assay 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H- tetrazolium-5-carboxanilide (XTT) and confocal laser scanning (a) Universidade Estadual Paulista – Unesp, microscopy (CLSM). Terpinen-4-ol and TTO inhibited C. albicans School of Dentistry of Araraquara, Department of Physiology and Pathology, growth. CLSM confirmed that 17.92 mg/mL of TTO and 8.86 mg/mL Araraquara, SP, Brazil of terpinen-4-ol applied for 60 s (rinse simulation) interfered with (b) Universidade Estadual de Campinas – biofilm formation. Hence, this in vitro study revealed that natural Unicamp, School of Dentistry of Piracicaba, substances such as TTO and terpinen-4-ol present promising results Department of Physiological Sciences, for the treatment of oral candidiasis. -

Antifungals, Topical
Therapeutic Class Overview Antifungals, Topical INTRODUCTION The topical antifungals are available in multiple dosage forms and are indicated for a number of fungal infections and related conditions. In general, these agents are Food and Drug Administration (FDA)-approved for the treatment of cutaneous candidiasis, onychomycosis, seborrheic dermatitis, tinea corporis, tinea cruris, tinea pedis, and tinea versicolor (Clinical Pharmacology 2018). The antifungals may be further classified into the following categories based upon their chemical structures: allylamines (naftifine, terbinafine [only available over the counter (OTC)]), azoles (clotrimazole, econazole, efinaconazole, ketoconazole, luliconazole, miconazole, oxiconazole, sertaconazole, sulconazole), benzylamines (butenafine), hydroxypyridones (ciclopirox), oxaborole (tavaborole), polyenes (nystatin), thiocarbamates (tolnaftate [no FDA-approved formulations]), and miscellaneous (undecylenic acid [no FDA-approved formulations]) (Micromedex 2018). The topical antifungals are available as single entity and/or combination products. Two combination products, nystatin/triamcinolone and Lotrisone (clotrimazole/betamethasone), contain an antifungal and a corticosteroid preparation. The corticosteroid helps to decrease inflammation and indirectly hasten healing time. The other combination product, Vusion (miconazole/zinc oxide/white petrolatum), contains an antifungal and zinc oxide. Zinc oxide acts as a skin protectant and mild astringent with weak antiseptic properties and helps to -

Oral Candidosis: Aetiology, Clinical Manifestations, Diagnosis and Management Birsay Gümrü Tarçın
MÜSBED 2011;1(2):140-148 Derleme / Review Oral Candidosis: Aetiology, Clinical Manifestations, Diagnosis and Management Birsay Gümrü Tarçın Marmara University Faculty of Dentistry, Department of Oral Diagnosis and Radiology, Istanbul-Turkey Ya zış ma Ad re si / Add ress rep rint re qu ests to: Birsay Gümrü Tarçın Marmara University Faculty of Dentistry, Department of Oral Diagnosis and Radiology, Büyükçiftlik Sok. No: 6 34365 Nişantaşı, Şişli, İstanbul-Turkey Telefon / Phone: +90-212-231-9120 Faks / Fax: +90-212-246-5247 Elekt ro nik pos ta ad re si / E-ma il add ress: [email protected] Ka bul ta ri hi / Da te of ac cep tan ce: 22 Ağustos 2011 / August 22, 2011 ÖZET ABS TRACT Oral kandidozis: Etiyoloji, klinik özellikler, tanı Oral candidosis: aetiology, clinical ve tedavi manifestations, diagnosis and management Oral kandidozis dişhekimliği pratiğinde en sık karşılaşılan fungal Oral candidosis is the most common fungal infection encountered enfeksiyondur. Birçok farklı klinik görünümde ortaya çıkabildi- in dental practice. Clinical diagnosis and management of oral ğinden, klinik tanı ve tedavisi genellikle zordur. Hastalık sıklıkla candidosis is usually complicated, because it is encountered in a sistemik rahatsızlığı olan spesifik hasta gruplarında ortaya çık- wide variety of clinical presentations. The disease often manifests maktadır. Bu nedenle, oral kandidozis tedavisi öncelikle predis- in specific patient groups that are systemically compromised. pozisyon yaratan durumların kapsamlı tetkikini içermelidir. Bu Therefore, the management should always cover a thorough derlemede sık karşılaşılan oral kandidal lezyonların etiyolojisi, investigation of underlying predisposing conditions. This klinik görünümü, tanı ve tedavi stratejileri kapsamlı bir şekilde review provides a comprehensive overview of the aetiology, gözden geçirilmektedir. -

Therapeutic Class Overview Antifungals, Topical
Therapeutic Class Overview Antifungals, Topical INTRODUCTION The topical antifungals are available in multiple dosage forms and are indicated for a number of fungal infections and related conditions. In general, these agents are Food and Drug Administration (FDA)-approved for the treatment of cutaneous candidiasis, onychomycosis, seborrheic dermatitis, tinea corporis, tinea cruris, tinea pedis, and tinea versicolor (Clinical Pharmacology 2018). The antifungals may be further classified into the following categories based upon their chemical structures: allylamines (naftifine, terbinafine [only available over the counter (OTC)]), azoles (clotrimazole, econazole, efinaconazole, ketoconazole, luliconazole, miconazole, oxiconazole, sertaconazole, sulconazole), benzylamines (butenafine), hydroxypyridones (ciclopirox), oxaborole (tavaborole), polyenes (nystatin), thiocarbamates (tolnaftate [no FDA-approved formulations]), and miscellaneous (undecylenic acid [no FDA-approved formulations]) (Micromedex 2018). The topical antifungals are available as single entity and/or combination products. Two combination products, nystatin/triamcinolone and Lotrisone (clotrimazole/betamethasone), contain an antifungal and a corticosteroid preparation. The corticosteroid helps to decrease inflammation and indirectly hasten healing time. The other combination product, Vusion (miconazole/zinc oxide/white petrolatum), contains an antifungal and zinc oxide. Zinc oxide acts as a skin protectant and mild astringent with weak antiseptic properties and helps to -

Therapeutic Drug Class
BUREAU FOR MEDICAL SERVICES WEST VIRGINIA MEDICAID EFFECTIVE PREFERRED DRUG LIST WITH PRIOR AUTHORIZATION CRITERIA 07/01/13 This is not an all-inclusive list of available covered drugs and includes only managed categories. Refer to cover page for complete list of rules governing this PDL. Version 2013.3c Prior authorization for a non-preferred agent in any category will be given only if there has been a trial of the preferred brand/generic equivalent or preferred formulation of the active ingredient, at a therapeutic dose, that resulted in a partial response with a documented intolerance. Prior authorization of a non-preferred isomer, pro-drug, or metabolite will be considered with a trial of a preferred parent drug of the same chemical entity, at a therapeutic dose, that resulted in a partial response with documented intolerance or a previous trial and therapy failure, at a therapeutic dose, with a preferred drug of a different chemical entity indicated to treat the submitted diagnosis. (The required trial may be overridden when documented evidence is provided that the use of these preferred agent(s) would be medically contraindicated.) Unless otherwise specified, the listing of a particular brand or generic name includes all legend forms of that drug. OTC drugs are not covered unless specified. PA criteria for non-preferred agents apply in addition to general Drug Utilization Review policy that is in effect for the entire pharmacy program, including, but not limited to, appropriate dosing, duplication of therapy, etc. The use of pharmaceutical samples will not be considered when evaluating the members’ medical condition or prior prescription history for drugs that require prior authorization. -

A Ketoconazole Susceptibility Test for Malassezia Pachydermatis Using Modified Leeming–Notman Agar
Journal of Fungi Article A Ketoconazole Susceptibility Test for Malassezia pachydermatis Using Modified Leeming–Notman Agar Bo-Young Hsieh 1,2, Wei-Hsun Chao 3, Yi-Jing Xue 2 and Jyh-Mirn Lai 2,* 1 Lugu Township Administration, Nanto County 55844, Taiwan; [email protected] 2 College of Veterinary Medicine, National Chiayi University, No. 580, XinMin Rd., Chiayi City 60054, Taiwan; [email protected] 3 Department of Hospitality Management, WuFung University, Chiayi City 60054, Taiwan; [email protected] * Correspondence: [email protected]; Tel.: +886-5-2732920; Fax: +886-5-2732917 Received: 18 September 2018; Accepted: 14 November 2018; Published: 16 November 2018 Abstract: The aim of this study was to establish a ketoconazole susceptibility test for Malassezia pachydermatis using modified Leeming–Notman agar (mLNA). The susceptibilities of 33 M. pachydermatis isolates obtained by modified CLSI M27-A3 method were compared with the results by disk diffusion method, which used different concentrations of ketoconazole on 6 mm diameter paper disks. Results showed that 93.9% (31/33) of the minimum inhibitory concentration (MIC) values obtained from both methods were similar (consistent with two methods within 2 dilutions). M. pachydermatis BCRC 21676 and Candida parapsilosis ATCC 22019 were used to verify the results obtained from the disk diffusion and modified CLSI M27-A3 tests, and they were found to be consistent. Therefore, the current study concludes that this new novel test—using different concentrations of reagents on cartridge disks to detect MIC values against ketoconazole—can be a cost-effective, time-efficient, and less technically demanding alternative to existing methods. -

Adverse Effects of Medications on Oral Health
Adverse Effects of Medications on Oral Health Dr. James Krebs, BS Pharm, MS, PharmD Director of Experiential Education College of Pharmacy, University of New England Presented by: Rachel Foster PharmD Candidate, Class of 2014 University of New England October 2013 Objectives • Describe the pathophysiology of various medication-related oral reactions • Recognize the signs and symptoms associated with medication-related oral reactions • Identify the populations associated with various offending agents • Compare the treatment options for medication-related oral reactions Medication-related Oral Reactions • Stomatitis • Oral Candidiasis • Burning mouth • Gingival hyperplasia syndrome • Alterations in • Glossitis salivation • Erythema • Alterations in taste Multiforme • Halitosis • Oral pigmentation • Angioedema • Tooth discoloration • Black hairy tongue Medication-related Stomatitis • Clinical presentation – Aphthous-like ulcers, mucositis, fixed-drug eruption, lichen planus1,2 – Open sores in the mouth • Tongue, gum line, buccal membrane – Patient complaint of soreness or burning http://www.virtualmedicalcentre.com/diseases/oral-mucositis-om/92 0 http://www.virtualmedicalcentre.com/diseases/oral-mucositis-om/920 Medication-related Stomatitis • Offending agents1,2 Medication Indication Patient Population Aspirin •Heart health • >18 years old •Pain reliever • Cardiac patients NSAIDs (i.e. Ibuprofen, •Headache General population naproxen) •Pain reliever •Fever reducer Chemotherapy (i.e. •Breast cancer •Oncology patients methotrexate, 5FU, •Colon -

Oral Manifestations of Pemphigus Vulgaris
Journal of Clinical & Experimental Dermatology Research - Open Access Research Article OPEN ACCESS Freely available online doi:10.4172/2155-9554.1000112 Oral Manifestations of Pemphigus Vulgaris: Clinical Presentation, Differential Diagnosis and Management Antonio Bascones-Martinez1*, Marta Munoz-Corcuera2, Cristina Bascones-Ilundain1 and German Esparza-Gómez1 1DDS, PhD, Medicine and Bucofacial Surgery Department, Dental School, Complutense University of Madrid, Spain 2DDS, PhD Student, Medicine and Bucofacial Surgery Department, Dental School, Complutense University of Madrid, Spain Abstract Pemphigus vulgaris is a chronic autoimmune mucocutaneous disease characterized by the formation of intraepithelial blisters. It results from an autoimmune process in which antibodies are produced against desmoglein 1 and desmoglein 3, normal components of the cell membrane of keratinocytes. The first manifestations of pemphigus vulgaris appear in the oral mucosa in the majority of patients, followed at a later date by cutaneous lesions. The diagnosis is based on clinical findings and laboratory analyses, and it is usually treated by the combined administration of corticosteroids and immunosuppressants. Detection of the oral lesions can result in an earlier diagnosis. We review the oral manifestations of pemphigus vulgaris as well as the differential diagnosis, treatment, and prognosis of oral lesions in this uncommon disease. Keywords: Pemphigus; Oral mucosa; Autoimmune bullous disease and have a molecular weight of 130 and 160 KDa, respectively [1,7,9,13]. The binding of antibodies to desmoglein at mucosal or Introduction cutaneous level gives rise to the loss of cell adhesion, with separation of epithelial layers (acantholysis) and the consequent appearance of Pemphigus vulgaris (PV) is the most frequently observed blisters on skin or mucosae [1,3]. -
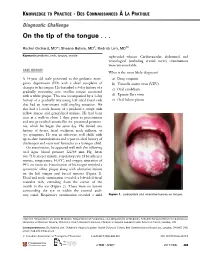
On the Tip of the Tongue
KNOWLEDGE TO PRACTICE DES CONNAISSANCES ÀLA PRATIQUE Diagnostic Challenge On the tip of the tongue . Rachel Orchard, MD*; Sheena Belisle, MD†; Rodrick Lim, MD†‡ Keywords: pediatric, rash, tongue, vesicle right-sided wheeze. Cardiovascular, abdominal, and neurological (including cranial nerve) examinations were unremarkable. CASE HISTORY What is the most likely diagnosis? A 14-year-old male presented to the pediatric emer- a) Drug eruption gency department (ED) with a chief complaint of b) Varicella zoster virus (VZV) changes to his tongue. He described a 3-day history of a c) Oral candidiasis gradually worsening sore, swollen tongue associated with a white plaque. This was accompanied by a 3-day d) Epstein-Barr virus history of a gradually worsening left-sided facial rash e) Oral lichen planus that had an intermittent mild tingling sensation. He also had a 1-week history of a productive cough with yellow mucus and generalized malaise. He had been seen at a walk-in clinic 2 days prior to presentation and was prescribed amoxicillin for presumed pneumo- nia, which he began the same day. He denied any history of fevers, facial weakness, neck stiffness, or eye symptoms. He was an otherwise well child, with up-to-date immunizations and a past medical history of chickenpox and recurrent furuncles as a younger child. On examination, he appeared well with the following vital signs: blood pressure 122/64 mm Hg, heart rate 73 beats per minute, respiratory rate 18 breaths per minute, temperature 36.8°C, and oxygen saturation of 99% on room air. Examination of his tongue revealed a symmetric white plaque along with ulcerative lesions on the left tongue and buccal mucosa (Figure 1). -

Abnormal Vaginal Discharge: What Does and Does Not Work in Treating Underlying Causes
AE_French.1104.final 10/18/04 11:03 AM Page 890 Applied Evidence N EW R ESEARCH F INDINGS T HAT A RE C HANGING C LINICAL P RACTICE Abnormal vaginal discharge: What does and does not work in treating underlying causes Linda French, MD Michigan State University, East Lansing, Mich Jennifer Horton, DO Genesys Regional Medical Center Family Practice Residency, Grand Blanc, Mich Michelle Matousek, DO Henry Ford Health System, Detroit, Mich Practice recommendations part of this article, “Abnormal vaginal discharge: Using office diagnostic testing more effectively” ■ Treat bacterial vaginosis with oral or intravagi- (JFP 2004; 53[10]:805–814), abnormal discharge nal metronidazole or with clindamycin (SOR: is more likely to be bacterial vaginosis or no A); recurrences are common (SOR: C). pathogen at all. Potential delay in diagnosis and treatment of a sexually transmitted disease is ■ Oral and intravaginal imidazoles are also a concern. Increasing resistance of Candida equally effective in the treatment of sp. to imidazoles is associated with indiscriminate candidiasis (SOR: A); alternate therapies use of over-the-counter products. for resistant cases have been little studied. ■ Oral metronidazole is the standard ■ BACTERIAL VAGINOSIS therapy for trichomoniasis (SOR: A). The standard treatment for bacterial vaginosis Oral tinidazole, newly available in the (BV) has been oral metronidazole (Flagyl) 500 mg US in 2004, should be used in resistant twice daily for 5 to 7 days. Intravaginal 0.75% cases (SOR: B). metronidazole gel (MetroGel) has been shown to be as effective as oral metronidazole (SOR: A).1,2 Oral metronidazole can cause nausea and ntifungal medications for intravaginal use abdominal pain in some patients; vaginal treat- have been available in the United States ment may be preferable for them. -

Antifungal and Antibiotic Powders
ANTIFUNGAL AND ANTIBIOTIC POWDERS Antifungals: Econazole Powder, Ketoconazole Powder, Nyamyc (nystatin) Powder, Nystop (nystatin) Powder Antibiotics: Mupirocin Powder, Tobramycin Powder, Vancomycin Powder RATIONALE FOR INCLUSION IN PA PROGRAM Background Pharmacy compounding is an ancient practice in which pharmacists combine, mix or alter ingredients to create unique medications that meet specific needs of individual patients. Some examples of the need for compounding products would be: the dosage formulation must be changed to allow a person with dysphagia (trouble swallowing) to have a liquid formulation of a commercially available tablet only product, or to obtain the exact strength needed of the active ingredient, to avoid ingredients that a particular patient has an allergy to, or simply to add flavoring to medication to make it more palatable. The intent of the criteria is to provide coverage consistent with product labeling, FDA guidance, standards of medical practice, evidence-based drug information, and/or published guidelines. Pharmacy powder products have the potential for misuse. Misuse of these powder products is quite common and it is important to inform patients about the possible complications due to overuse of these drugs. Regulatory Status FDA approved indication: Antifungal agents kill fungi or inhibit their growth. Antifungals that kill fungi are called fungicidal while those that inhibit their growth are called fungistatic. Antibiotics, or antimicrobials, are medications that destroy or slow down the growth of bacteria. -

New Mechanism of Oral Immunity to Mucosal Candidiasis in Hyper-Ige Syndrome
ARTICLES nature publishing group New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome H R C o n t i 1 , 4 , O B a k e r 1 , A F F r e e m a n 2 , W S J a n g 1 , S M H o l l a n d 2 , R A L i 1 , M E d g e r t o n 1 a n d S L G a f f e n 1 , 3 Oropharyngeal candidiasis (OPC, thrush) is an opportunistic infection caused by the commensal fungus Candida albicans . An understanding of immunity to Candida has recently begun to unfold with the identification of fungal pattern- recognition receptors such as C-type lectin receptors, which trigger protective T-helper (Th)17 responses in the mucosa. Hyper-IgE syndrome (HIES / Job ’ s syndrome) is a rare congenital immunodeficiency characterized by dominant-negative mutations in signal transducer and activator of transcription 3, which is downstream of the Th17-inductive cytokines interleukin (IL)-6 and IL-23, and hence patients with HIES exhibit dramatic Th17 deficits. HIES patients develop oral and mucocutaneous candidiasis, supporting a protective role for Th17 cells in immunity to OPC. However, the Th17- dependent mechanisms of antifungal immunity in OPC are still poorly defined. An often unappreciated aspect of oral immunity is saliva, which is rich in antimicrobial proteins (AMPs) and exerts direct antifungal activity. In this study, we show that HIES patients show significant impairment in salivary AMPs, including -defensin 2 and Histatins. This tightly correlates with reduced candidacidal activity of saliva and concomitantly elevated colonization with Candida .